

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183044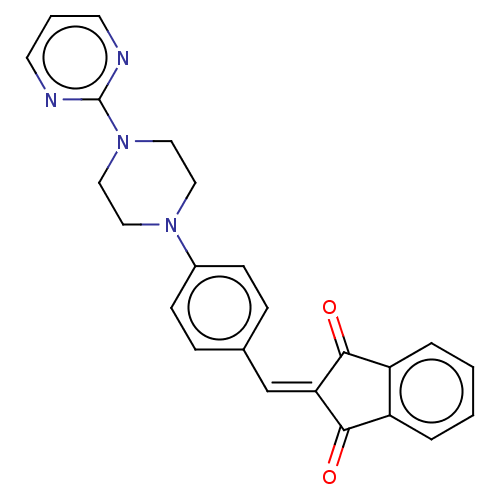 (CHEMBL3818053) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183042 (CHEMBL3818600) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183043 (CHEMBL3819454) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183046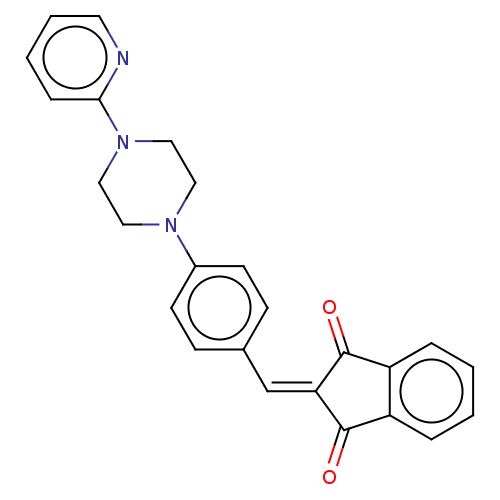 (CHEMBL3817925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183047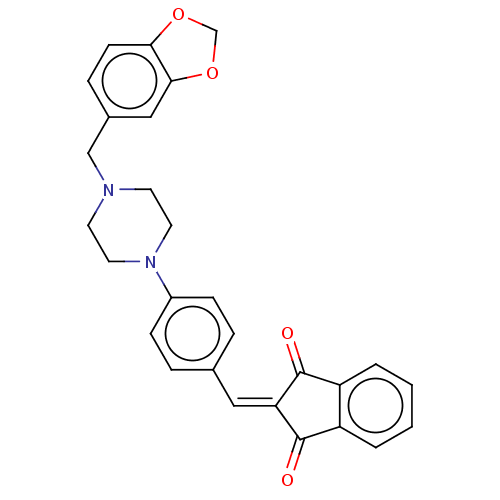 (CHEMBL3819248) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183052 (CHEMBL3819316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183053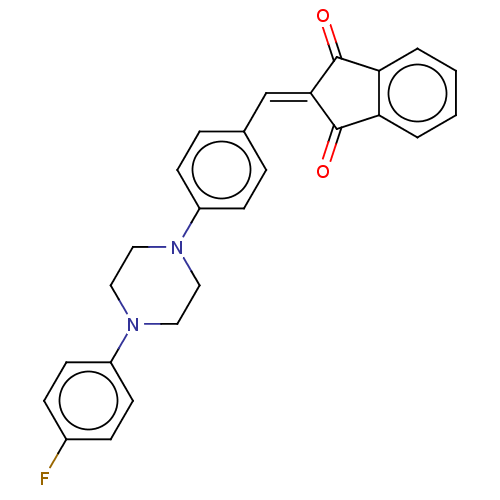 (CHEMBL3819571) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183051 (CHEMBL3819042) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183050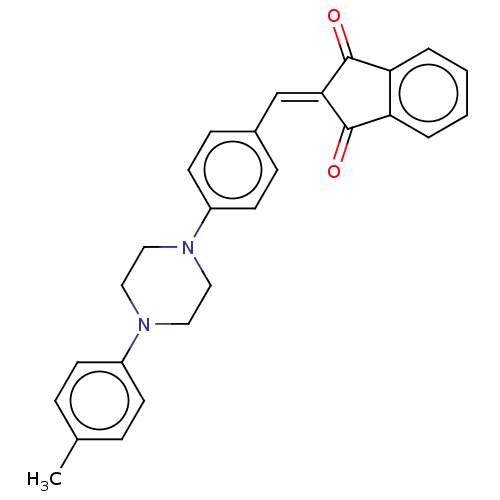 (CHEMBL3818518) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183049 (CHEMBL3819593) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183050 (CHEMBL3818518) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183049 (CHEMBL3819593) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183046 (CHEMBL3817925) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183051 (CHEMBL3819042) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183052 (CHEMBL3819316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183054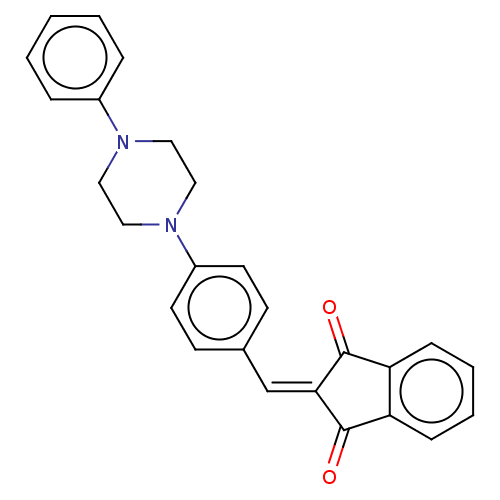 (CHEMBL3818978) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183042 (CHEMBL3818600) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183044 (CHEMBL3818053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183043 (CHEMBL3819454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183047 (CHEMBL3819248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183054 (CHEMBL3818978) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183053 (CHEMBL3819571) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50183042 (CHEMBL3818600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of self-induced amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs measured after 5 mins by thioflavin-T fluorescenc... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50183044 (CHEMBL3818053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of self-induced amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs measured after 5 mins by thioflavin-T fluorescenc... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50183043 (CHEMBL3819454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of self-induced amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs measured after 5 mins by thioflavin-T fluorescenc... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50183048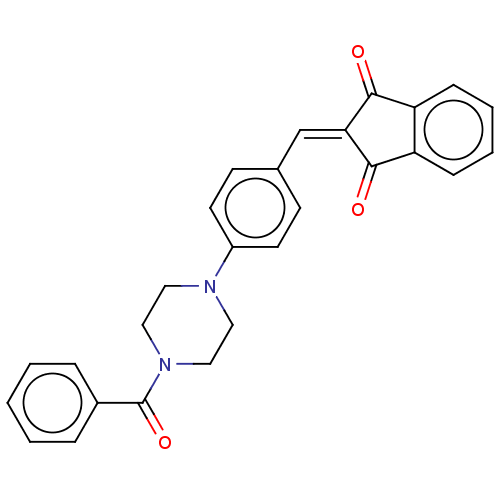 (CHEMBL3818091) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50183048 (CHEMBL3818091) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Inhibition of self-induced amyloid beta (1 to 42) (unknown origin) aggregation incubated for 48 hrs measured after 5 mins by thioflavin-T fluorescenc... | Bioorg Med Chem 24: 3829-41 (2016) Article DOI: 10.1016/j.bmc.2016.06.027 BindingDB Entry DOI: 10.7270/Q24Q7WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||