 Found 28 hits of Enzyme Inhibition Constant Data
Found 28 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207291
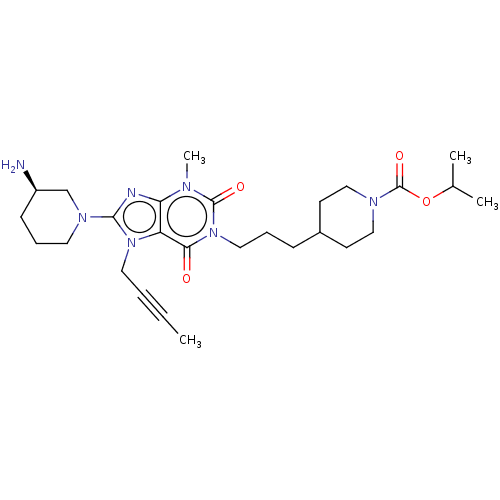
(CHEMBL3902771)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCC3CCN(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C27H41N7O4/c1-5-6-14-33-22-23(29-25(33)32-13-8-10-21(28)18-32)30(4)26(36)34(24(22)35)15-7-9-20-11-16-31(17-12-20)27(37)38-19(2)3/h19-21H,7-18,28H2,1-4H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207295
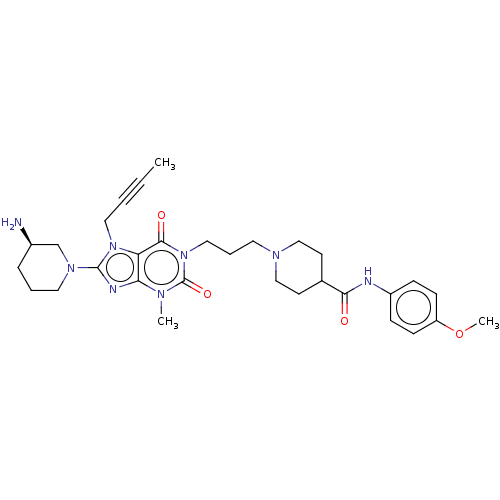
(CHEMBL3984849)Show SMILES COc1ccc(NC(=O)C2CCN(CCCn3c(=O)n(C)c4nc(N5CCC[C@@H](N)C5)n(CC#CC)c4c3=O)CC2)cc1 |r| Show InChI InChI=1S/C31H42N8O4/c1-4-5-17-38-26-27(34-30(38)37-16-6-8-23(32)21-37)35(2)31(42)39(29(26)41)18-7-15-36-19-13-22(14-20-36)28(40)33-24-9-11-25(43-3)12-10-24/h9-12,22-23H,6-8,13-21,32H2,1-3H3,(H,33,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207300
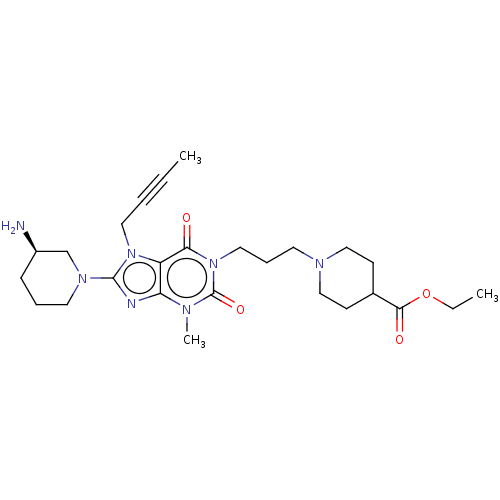
(CHEMBL3980269)Show SMILES CCOC(=O)C1CCN(CCCn2c(=O)n(C)c3nc(N4CCC[C@@H](N)C4)n(CC#CC)c3c2=O)CC1 |r| Show InChI InChI=1S/C26H39N7O4/c1-4-6-14-32-21-22(28-25(32)31-13-7-9-20(27)18-31)29(3)26(36)33(23(21)34)15-8-12-30-16-10-19(11-17-30)24(35)37-5-2/h19-20H,5,7-18,27H2,1-3H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207293

(CHEMBL3892718)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C27H41N7O4/c1-5-6-14-33-22-23(29-26(33)32-13-7-9-21(28)18-32)30(4)27(37)34(24(22)35)15-8-12-31-16-10-20(11-17-31)25(36)38-19(2)3/h19-21H,7-18,28H2,1-4H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207277

(CHEMBL3976778)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccccc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H40N8O3/c1-3-4-17-37-25-26(33-29(37)36-16-8-10-23(31)21-36)34(2)30(41)38(28(25)40)18-9-15-35-19-13-22(14-20-35)27(39)32-24-11-6-5-7-12-24/h5-7,11-12,22-23H,8-10,13-21,31H2,1-2H3,(H,32,39)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207287
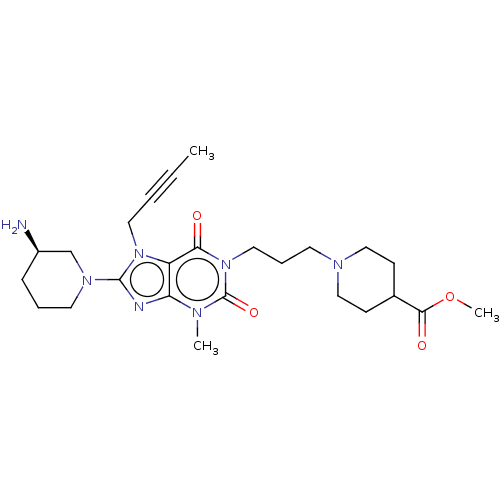
(CHEMBL3910158)Show SMILES COC(=O)C1CCN(CCCn2c(=O)n(C)c3nc(N4CCC[C@@H](N)C4)n(CC#CC)c3c2=O)CC1 |r| Show InChI InChI=1S/C25H37N7O4/c1-4-5-13-31-20-21(27-24(31)30-12-6-8-19(26)17-30)28(2)25(35)32(22(20)33)14-7-11-29-15-9-18(10-16-29)23(34)36-3/h18-19H,6-17,26H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207290

(CHEMBL3901674)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCCC3CCN(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C28H43N7O4/c1-5-6-15-34-23-24(30-26(34)33-14-9-11-22(29)19-33)31(4)27(37)35(25(23)36)16-8-7-10-21-12-17-32(18-13-21)28(38)39-20(2)3/h20-22H,7-19,29H2,1-4H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207283

(CHEMBL3897118)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)N(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C26H40N8O3/c1-5-6-14-33-21-22(28-25(33)32-13-7-9-20(27)18-32)30(4)26(37)34(24(21)36)15-8-12-31-16-10-19(11-17-31)23(35)29(2)3/h19-20H,7-18,27H2,1-4H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207294

(CHEMBL3968249)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Oc3ccccc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H39N7O4/c1-3-4-17-36-25-26(32-29(36)35-16-8-10-23(31)21-35)33(2)30(40)37(27(25)38)18-9-15-34-19-13-22(14-20-34)28(39)41-24-11-6-5-7-12-24/h5-7,11-12,22-23H,8-10,13-21,31H2,1-2H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207278
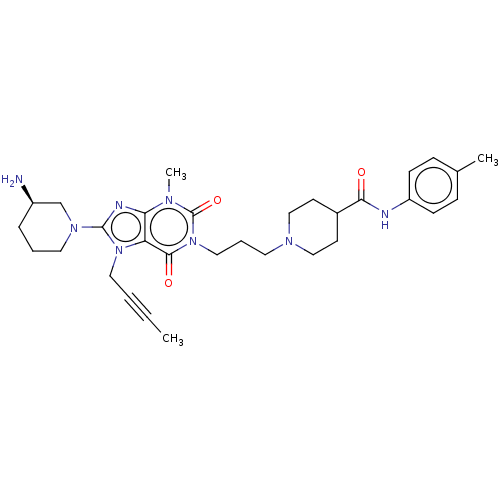
(CHEMBL3898220)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccc(C)cc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C31H42N8O3/c1-4-5-17-38-26-27(34-30(38)37-16-6-8-24(32)21-37)35(3)31(42)39(29(26)41)18-7-15-36-19-13-23(14-20-36)28(40)33-25-11-9-22(2)10-12-25/h9-12,23-24H,6-8,13-21,32H2,1-3H3,(H,33,40)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207279
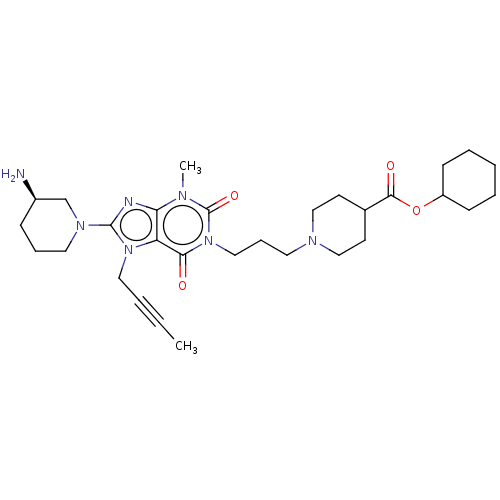
(CHEMBL3979866)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC3CCCCC3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H45N7O4/c1-3-4-17-36-25-26(32-29(36)35-16-8-10-23(31)21-35)33(2)30(40)37(27(25)38)18-9-15-34-19-13-22(14-20-34)28(39)41-24-11-6-5-7-12-24/h22-24H,5-21,31H2,1-2H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207281

(CHEMBL3896047)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccc(Cl)cc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H39ClN8O3/c1-3-4-16-38-25-26(34-29(38)37-15-5-7-23(32)20-37)35(2)30(42)39(28(25)41)17-6-14-36-18-12-21(13-19-36)27(40)33-24-10-8-22(31)9-11-24/h8-11,21,23H,5-7,12-20,32H2,1-2H3,(H,33,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207289
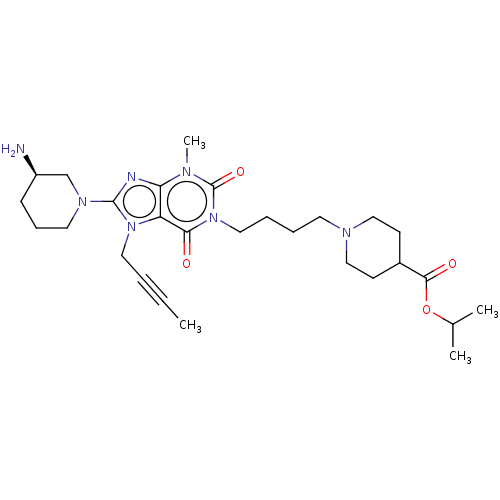
(CHEMBL3920718)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCCN3CCC(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C28H43N7O4/c1-5-6-15-34-23-24(30-27(34)33-14-9-10-22(29)19-33)31(4)28(38)35(25(23)36)16-8-7-13-32-17-11-21(12-18-32)26(37)39-20(2)3/h20-22H,7-19,29H2,1-4H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207286
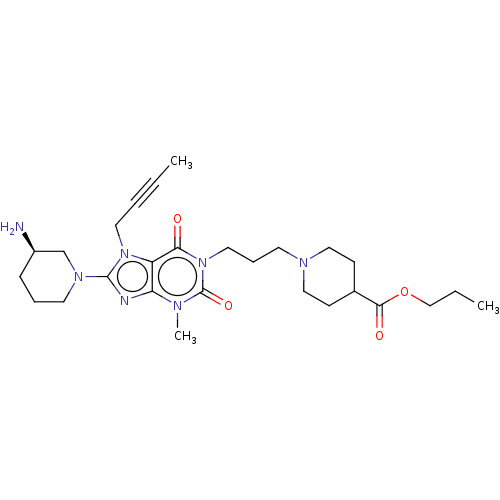
(CHEMBL3933623)Show SMILES CCCOC(=O)C1CCN(CCCn2c(=O)n(C)c3nc(N4CCC[C@@H](N)C4)n(CC#CC)c3c2=O)CC1 |r| Show InChI InChI=1S/C27H41N7O4/c1-4-6-14-33-22-23(29-26(33)32-13-7-9-21(28)19-32)30(3)27(37)34(24(22)35)15-8-12-31-16-10-20(11-17-31)25(36)38-18-5-2/h20-21H,5,7-19,28H2,1-3H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207282
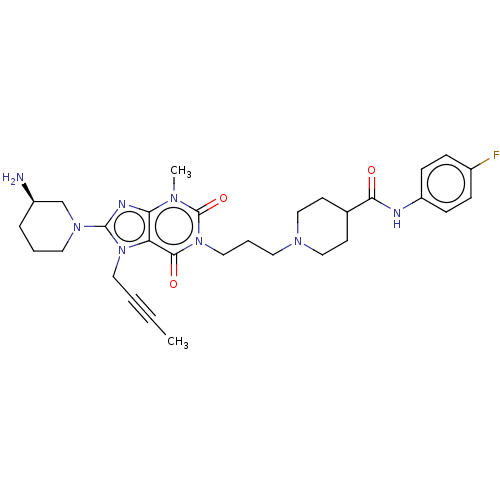
(CHEMBL3915044)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccc(F)cc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H39FN8O3/c1-3-4-16-38-25-26(34-29(38)37-15-5-7-23(32)20-37)35(2)30(42)39(28(25)41)17-6-14-36-18-12-21(13-19-36)27(40)33-24-10-8-22(31)9-11-24/h8-11,21,23H,5-7,12-20,32H2,1-2H3,(H,33,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207285
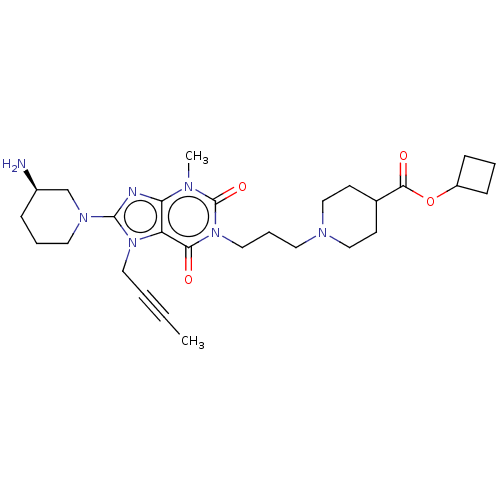
(CHEMBL3972227)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC3CCC3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C28H41N7O4/c1-3-4-15-34-23-24(30-27(34)33-14-6-8-21(29)19-33)31(2)28(38)35(25(23)36)16-7-13-32-17-11-20(12-18-32)26(37)39-22-9-5-10-22/h20-22H,5-19,29H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207296
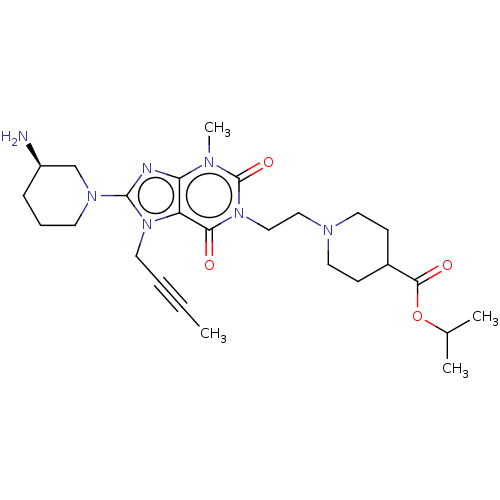
(CHEMBL3929627)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCN3CCC(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C26H39N7O4/c1-5-6-12-32-21-22(28-25(32)31-11-7-8-20(27)17-31)29(4)26(36)33(23(21)34)16-15-30-13-9-19(10-14-30)24(35)37-18(2)3/h18-20H,7-17,27H2,1-4H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207299
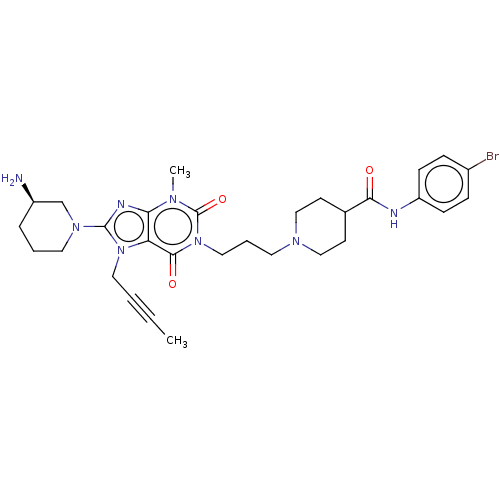
(CHEMBL3924059)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccc(Br)cc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H39BrN8O3/c1-3-4-16-38-25-26(34-29(38)37-15-5-7-23(32)20-37)35(2)30(42)39(28(25)41)17-6-14-36-18-12-21(13-19-36)27(40)33-24-10-8-22(31)9-11-24/h8-11,21,23H,5-7,12-20,32H2,1-2H3,(H,33,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207280
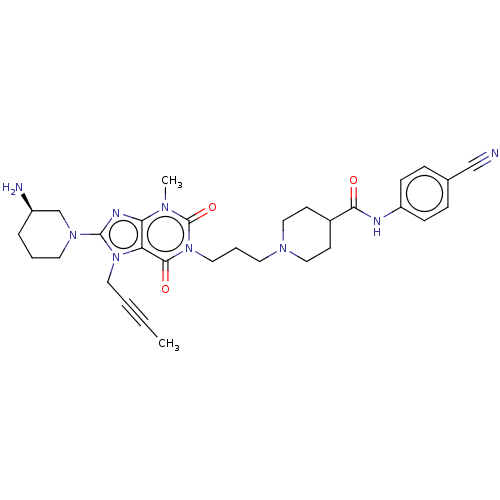
(CHEMBL3945617)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccc(cc3)C#N)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C31H39N9O3/c1-3-4-16-39-26-27(35-30(39)38-15-5-7-24(33)21-38)36(2)31(43)40(29(26)42)17-6-14-37-18-12-23(13-19-37)28(41)34-25-10-8-22(20-32)9-11-25/h8-11,23-24H,5-7,12-19,21,33H2,1-2H3,(H,34,41)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207298
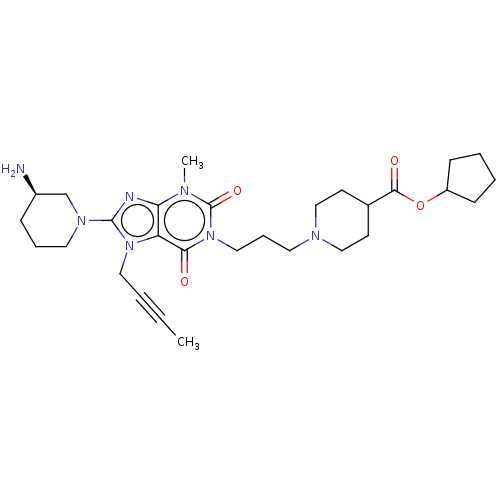
(CHEMBL3899590)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC3CCCC3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C29H43N7O4/c1-3-4-16-35-24-25(31-28(35)34-15-7-9-22(30)20-34)32(2)29(39)36(26(24)37)17-8-14-33-18-12-21(13-19-33)27(38)40-23-10-5-6-11-23/h21-23H,5-20,30H2,1-2H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207297
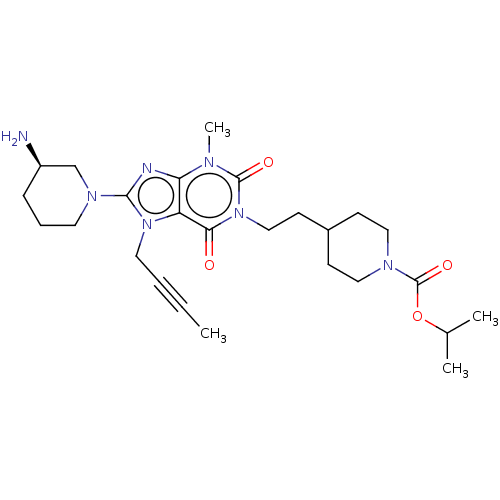
(CHEMBL3973741)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCC3CCN(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C26H39N7O4/c1-5-6-13-32-21-22(28-24(32)31-12-7-8-20(27)17-31)29(4)25(35)33(23(21)34)16-11-19-9-14-30(15-10-19)26(36)37-18(2)3/h18-20H,7-17,27H2,1-4H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207284
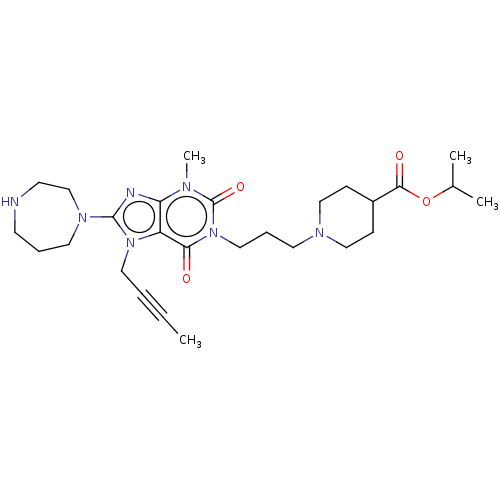
(CHEMBL3927979)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC(C)C)c(=O)c12)N1CCCNCC1 Show InChI InChI=1S/C27H41N7O4/c1-5-6-15-33-22-23(29-26(33)32-14-7-11-28-12-19-32)30(4)27(37)34(24(22)35)16-8-13-31-17-9-21(10-18-31)25(36)38-20(2)3/h20-21,28H,7-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207292
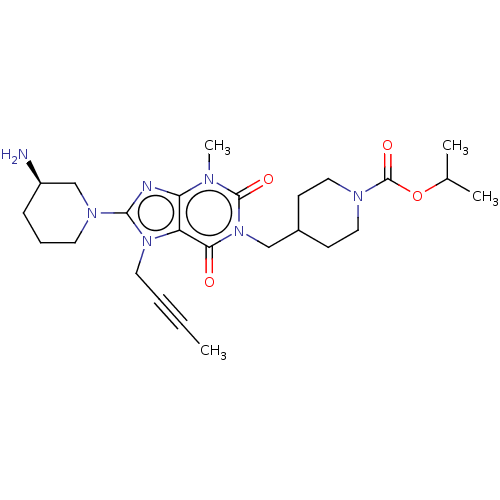
(CHEMBL3911786)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CC3CCN(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H37N7O4/c1-5-6-12-31-20-21(27-23(31)30-11-7-8-19(26)16-30)28(4)24(34)32(22(20)33)15-18-9-13-29(14-10-18)25(35)36-17(2)3/h17-19H,7-16,26H2,1-4H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50207288
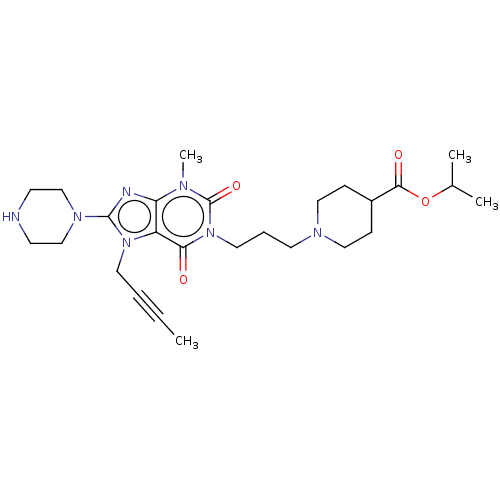
(CHEMBL3909049)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)OC(C)C)c(=O)c12)N1CCNCC1 Show InChI InChI=1S/C26H39N7O4/c1-5-6-13-32-21-22(28-25(32)31-17-10-27-11-18-31)29(4)26(36)33(23(21)34)14-7-12-30-15-8-20(9-16-30)24(35)37-19(2)3/h19-20,27H,7-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-p-nitroanilide as substrate incubated for 1 hr |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50207292

(CHEMBL3911786)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CC3CCN(CC3)C(=O)OC(C)C)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H37N7O4/c1-5-6-12-31-20-21(27-23(31)30-11-7-8-19(26)16-30)28(4)24(34)32(22(20)33)15-18-9-13-29(14-10-18)25(35)36-17(2)3/h17-19H,7-16,26H2,1-4H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Agonist activity at CREB-LBD and GAL4-DBD fused human GPR119 expressed in HEK293 cells by luciferase reporter gene assay |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50244791
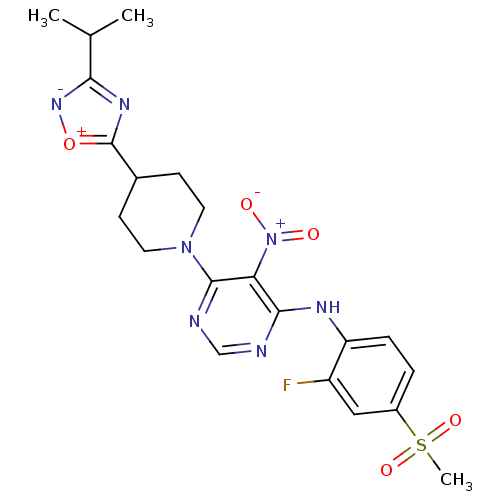
((2-Fluoro-4-methanesulfonyl-phenyl)-{6-[4-(3-isopr...)Show SMILES CC(C)c1nc([o+][n-]1)C1CCN(CC1)c1ncnc(Nc2ccc(cc2F)S(C)(=O)=O)c1[N+]([O-])=O Show InChI InChI=1S/C21H24FN7O5S/c1-12(2)18-26-21(34-27-18)13-6-8-28(9-7-13)20-17(29(30)31)19(23-11-24-20)25-16-5-4-14(10-15(16)22)35(3,32)33/h4-5,10-13H,6-9H2,1-3H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Agonist activity at CREB-LBD and GAL4-DBD fused human GPR119 expressed in HEK293 cells by luciferase reporter gene assay |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50207277

(CHEMBL3976778)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CCCN3CCC(CC3)C(=O)Nc3ccccc3)c(=O)c12)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C30H40N8O3/c1-3-4-17-37-25-26(33-29(37)36-16-8-10-23(31)21-36)34(2)30(41)38(28(25)40)18-9-15-35-19-13-22(14-20-35)27(39)32-24-11-6-5-7-12-24/h5-7,11-12,22-23H,8-10,13-21,31H2,1-2H3,(H,32,39)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Agonist activity at CREB-LBD and GAL4-DBD fused human GPR119 expressed in HEK293 cells by luciferase reporter gene assay |
Eur J Med Chem 124: 103-116 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.023
BindingDB Entry DOI: 10.7270/Q2FJ2JRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data