

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP19 expressed in baculovirus infected insect cells using MFC as substrate measured after 30 mins by fluorometric an... | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197072 (CHEMBL3948254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197070 (CHEMBL3944017) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197069 (CHEMBL3905734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197071 (CHEMBL3350384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197076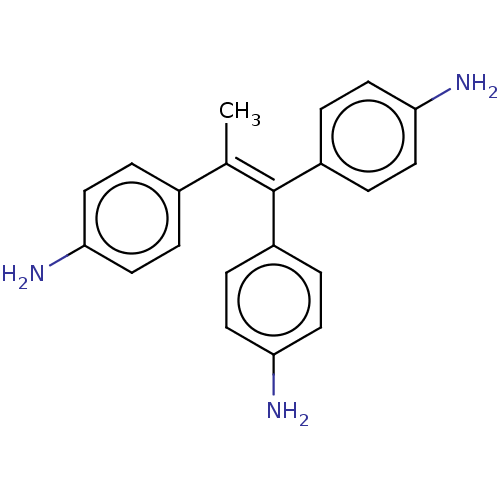 (CHEMBL3916057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197081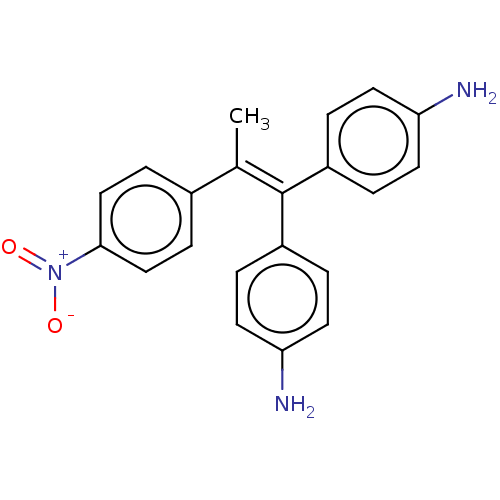 (CHEMBL3984531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197078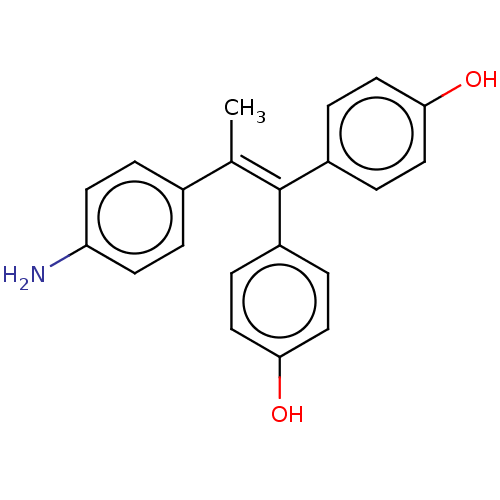 (CHEMBL3977491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197077 (CHEMBL3961452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197075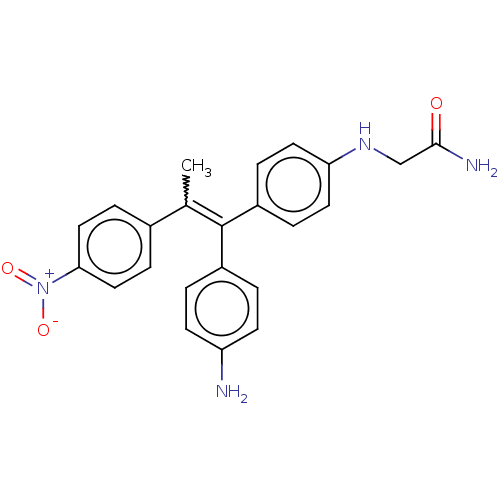 (CHEMBL3961033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197080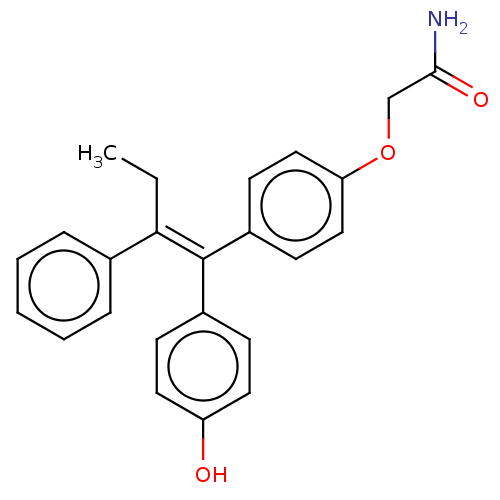 (CHEMBL3942918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50197068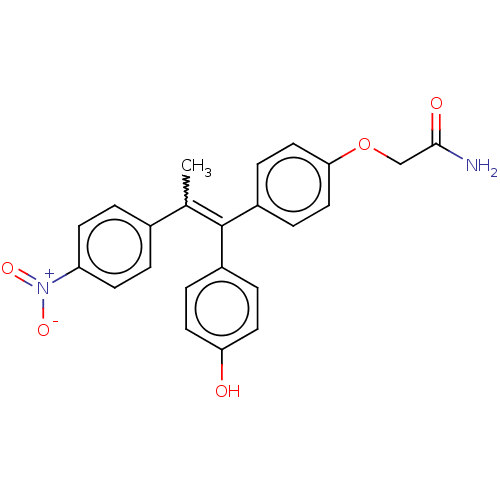 (CHEMBL3925065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50121317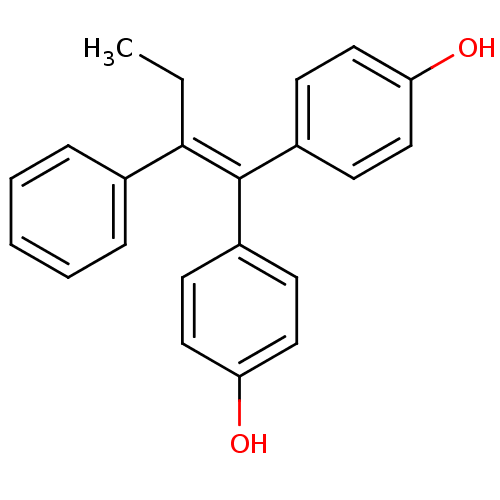 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197069 (CHEMBL3905734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197072 (CHEMBL3948254) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197078 (CHEMBL3977491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197071 (CHEMBL3350384) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197075 (CHEMBL3961033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 164 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197077 (CHEMBL3961452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 451 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197075 (CHEMBL3961033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 218 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197072 (CHEMBL3948254) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197069 (CHEMBL3905734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197077 (CHEMBL3961452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 346 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197081 (CHEMBL3984531) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 213 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197081 (CHEMBL3984531) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 486 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50197071 (CHEMBL3350384) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50197078 (CHEMBL3977491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 857 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||