

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236331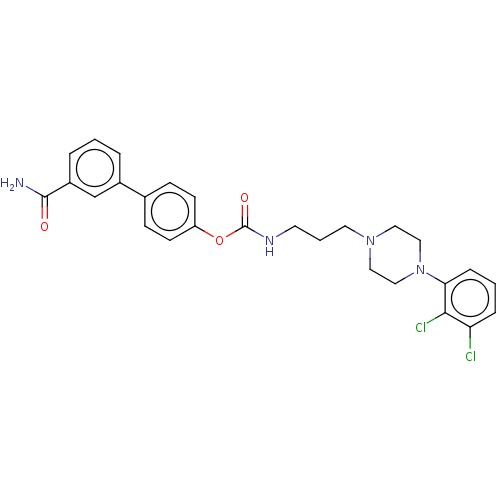 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236330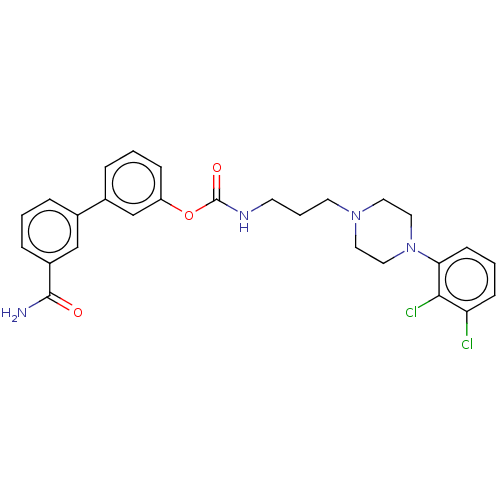 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236333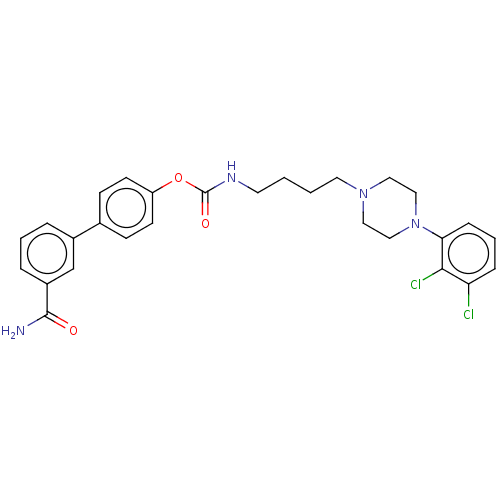 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236340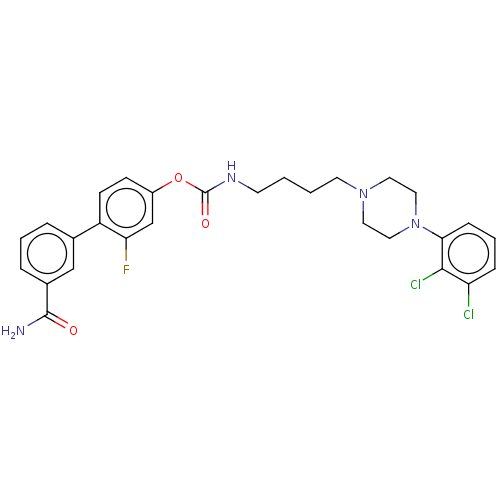 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236326 (CHEMBL4073966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236342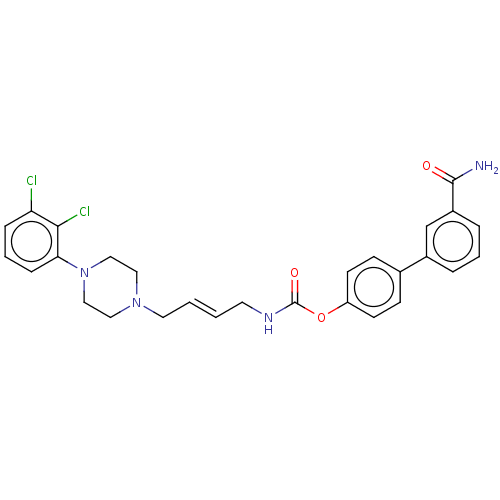 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236327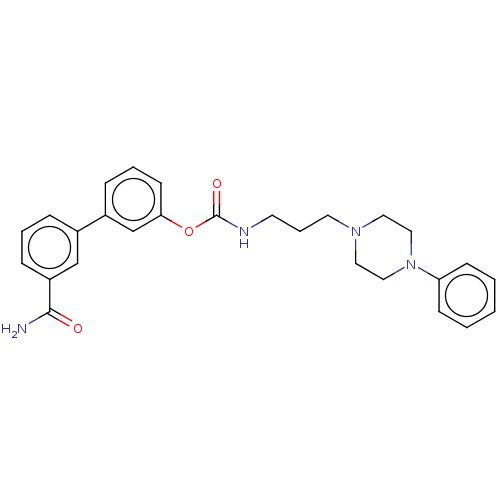 (CHEMBL4100735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1a adrenergic receptor | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236334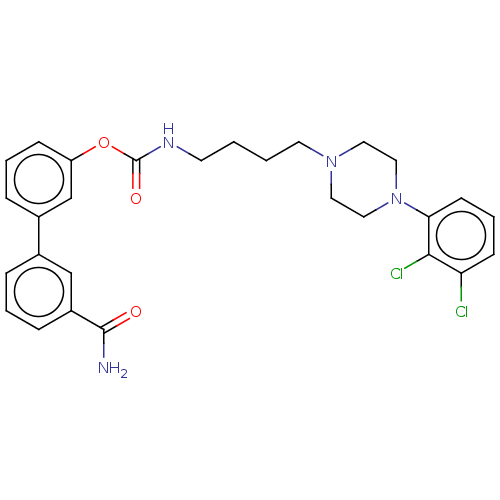 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236328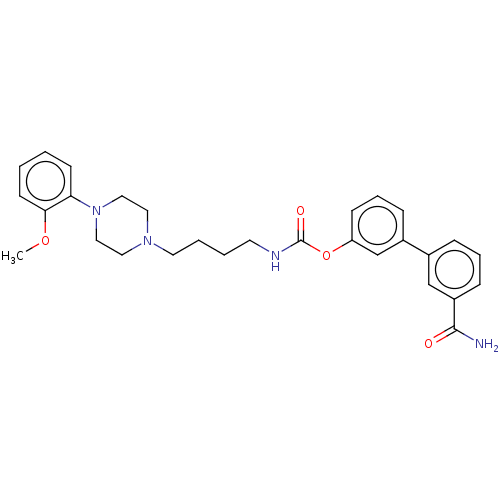 (CHEMBL4099798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236356 (CHEMBL4095588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236357 (CHEMBL4078351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236353 (CHEMBL4060896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236332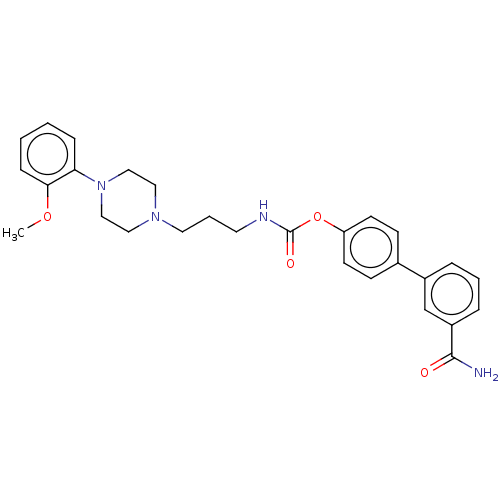 (CHEMBL4072282) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236339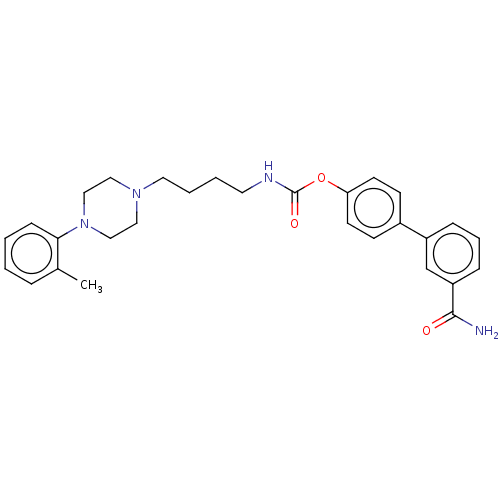 (CHEMBL4094794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236344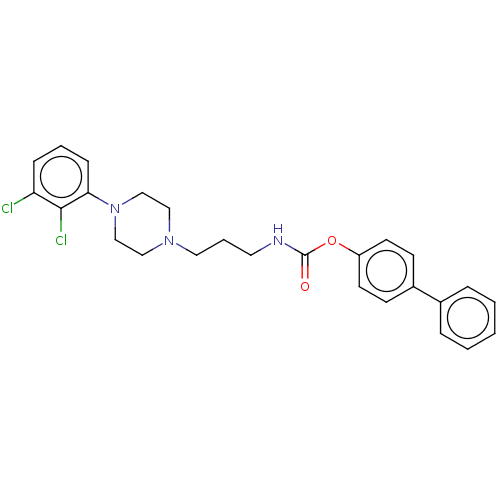 (CHEMBL4103339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236352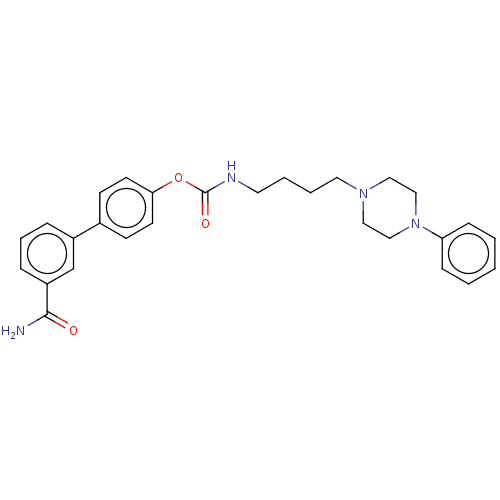 (CHEMBL4090581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236358 (CHEMBL4063681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236337 (CHEMBL4098174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]anandamide from FAAH in rat brain membranes after 30 mins by liquid scintillation counting | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236348 (CHEMBL4092900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236336 (CHEMBL4062484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236354 (CHEMBL4087889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236349 (CHEMBL4072140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236338 (CHEMBL4099236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236351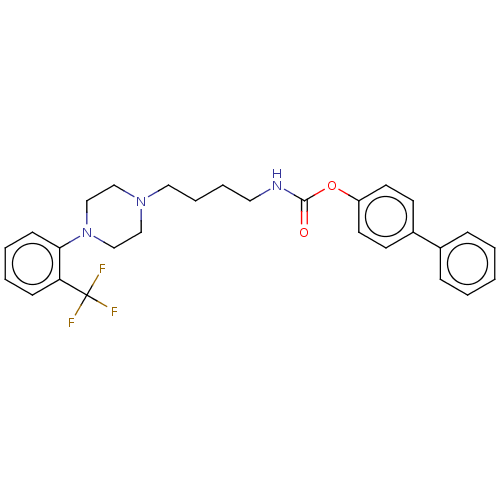 (CHEMBL4059573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236350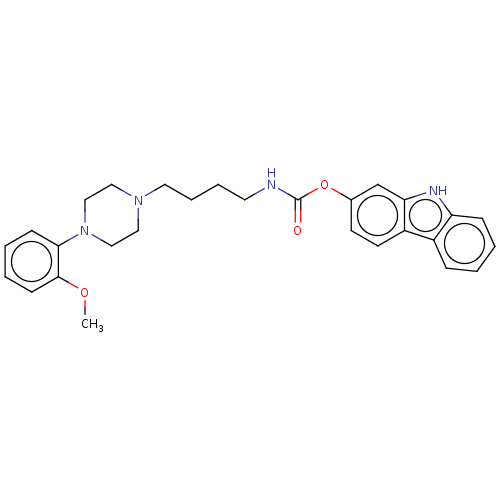 (CHEMBL4071121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Ability to displace [3H]-SR- 141716A binding to human CB1 receptor expressed in CHO cell membranes in absence of agonist 5''-guanylyimidodiphosphate | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236355 (CHEMBL4068218) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236335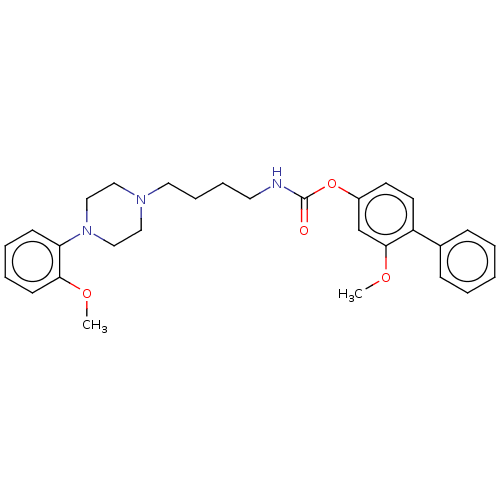 (CHEMBL4090459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236341 (CHEMBL4102620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50128585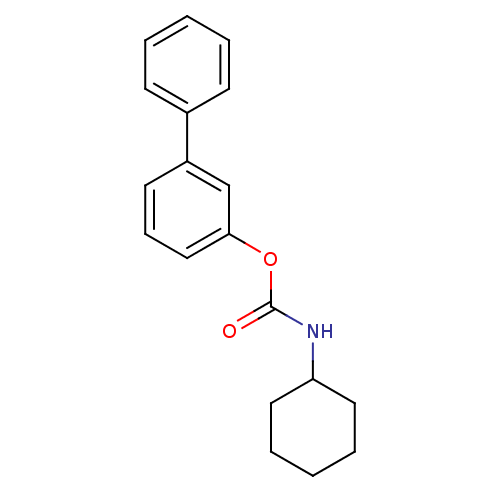 (CHEMBL431202 | Cyclohexyl-carbamic acid biphenyl-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]anandamide from FAAH in rat brain membranes after 30 mins by liquid scintillation counting | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236333 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236346 (CHEMBL4100634) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236349 (CHEMBL4072140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236335 (CHEMBL4090459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236337 (CHEMBL4098174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236341 (CHEMBL4102620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236348 (CHEMBL4092900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236334 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236355 (CHEMBL4068218) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236350 (CHEMBL4071121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236336 (CHEMBL4062484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 339 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236353 (CHEMBL4060896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro thrombin inhibition | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236338 (CHEMBL4099236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236331 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236326 (CHEMBL4073966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236342 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236347 (CHEMBL4082631) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 667 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236350 (CHEMBL4071121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236340 (CHEMBL4069565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236332 (CHEMBL4072282) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236332 (CHEMBL4072282) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236334 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236332 (CHEMBL4072282) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236339 (CHEMBL4094794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236333 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236340 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236344 (CHEMBL4103339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236328 (CHEMBL4099798) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236331 (CHEMBL4091498) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236349 (CHEMBL4072140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236358 (CHEMBL4063681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236340 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236328 (CHEMBL4099798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236330 (CHEMBL4070196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236334 (CHEMBL4071240) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236338 (CHEMBL4099236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236349 (CHEMBL4072140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 372 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236344 (CHEMBL4103339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236342 (CHEMBL4065510) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 147 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236337 (CHEMBL4098174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236352 (CHEMBL4090581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236339 (CHEMBL4094794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236344 (CHEMBL4103339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236334 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236330 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236328 (CHEMBL4099798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236331 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236332 (CHEMBL4072282) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236339 (CHEMBL4094794) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236344 (CHEMBL4103339) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236351 (CHEMBL4059573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236357 (CHEMBL4078351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236333 (CHEMBL4092052) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236338 (CHEMBL4099236) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236348 (CHEMBL4092900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236330 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236356 (CHEMBL4095588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236328 (CHEMBL4099798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236342 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236350 (CHEMBL4071121) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro thrombin inhibition | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236335 (CHEMBL4090459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236338 (CHEMBL4099236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236341 (CHEMBL4102620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236350 (CHEMBL4071121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236336 (CHEMBL4062484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236336 (CHEMBL4062484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236342 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236327 (CHEMBL4100735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236331 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236330 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236339 (CHEMBL4094794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM84889 (THC, 11-OH-delta 8 | THC-DMH, (+)-11-OH) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236337 (CHEMBL4098174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236348 (CHEMBL4092900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236341 (CHEMBL4102620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor-short expressed in CHO-K1 cells assessed as reduction in adenylyl cyclase activator NKH 477 induced cA... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236354 (CHEMBL4087889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human dopamine D3 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 30 mins by HTRF ... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236340 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236341 (CHEMBL4102620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236335 (CHEMBL4090459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236333 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Agonist activity at recombinant human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP accumulation after 20 mins by HTRF assay | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||