

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235301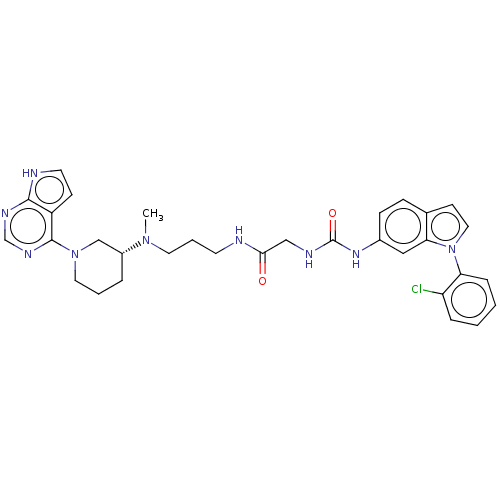 (CHEMBL4081752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISA | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235299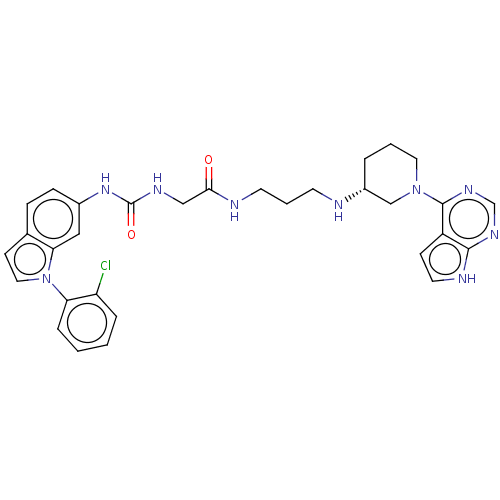 (CHEMBL4066397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235300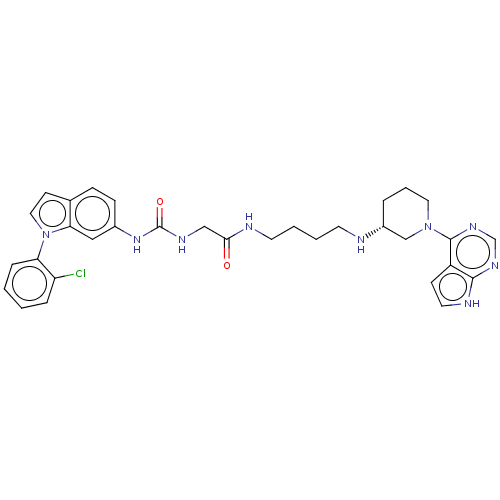 (CHEMBL4087730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235299 (CHEMBL4066397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235300 (CHEMBL4087730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISA | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L in human MOLM13 cells assessed as suppression of HoxA9 gene after 72 hrs by luciferase reporter gene assay | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L in human MOLM13 cells assessed as suppression of HoxA9 gene after 72 hrs by luciferase reporter gene assay | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235303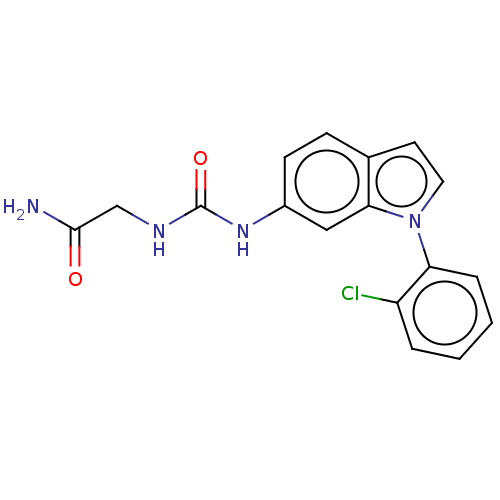 (CHEMBL4095429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235304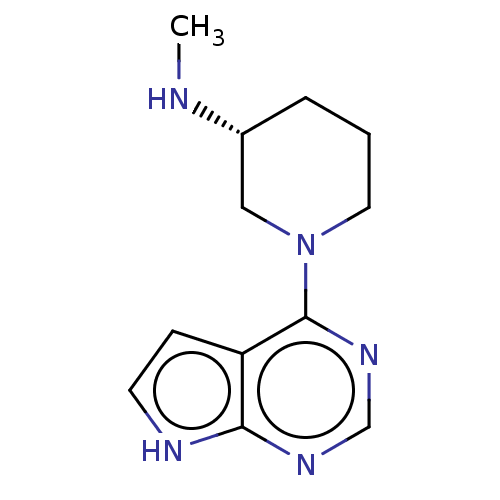 (CHEMBL4091413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235298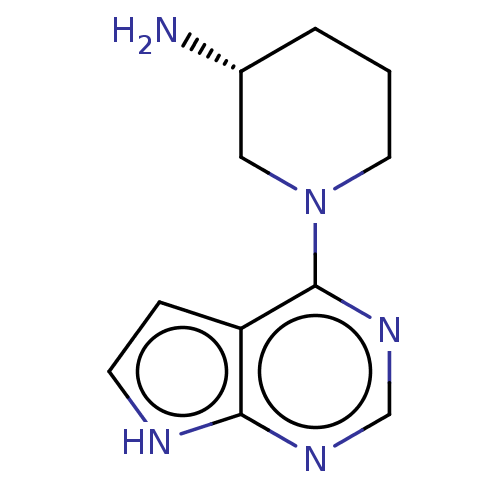 (CHEMBL4074998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235298 (CHEMBL4074998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||