

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50241979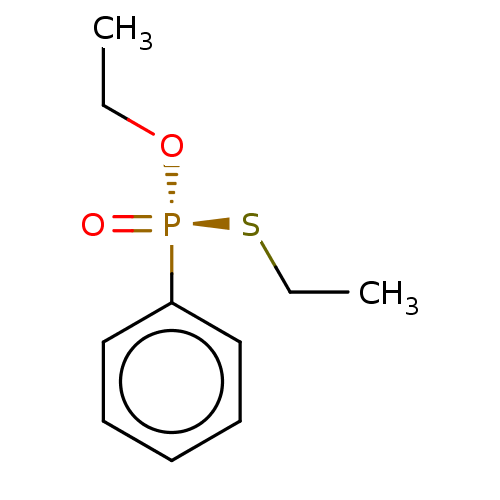 (CHEMBL4066365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of equine BChE assessed as equilibrium constant using butyrylthiocholine iodide as substrate after 30 mins | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241979 (CHEMBL4066365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as equilibrium constant using acetylthiocholine as substrate after 30 mins | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50240416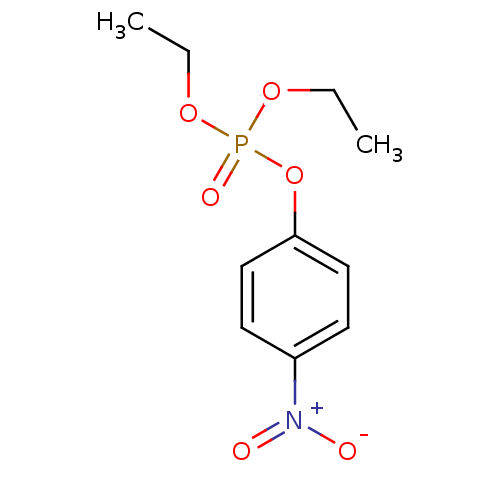 (CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50240416 (CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE using p-nitrophenyl acetate as substrate measured for 30 secs by spectrophotometric analysis | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241989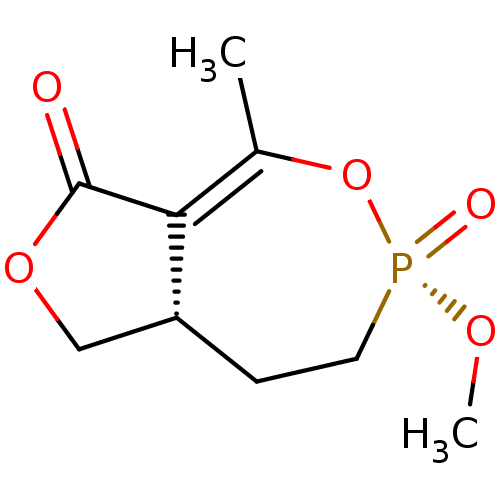 (CHEMBL2179375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 30 min followed by substrate addition measured ever... | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241979 (CHEMBL4066365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of horse BChE using butyrylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241979 (CHEMBL4066365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241988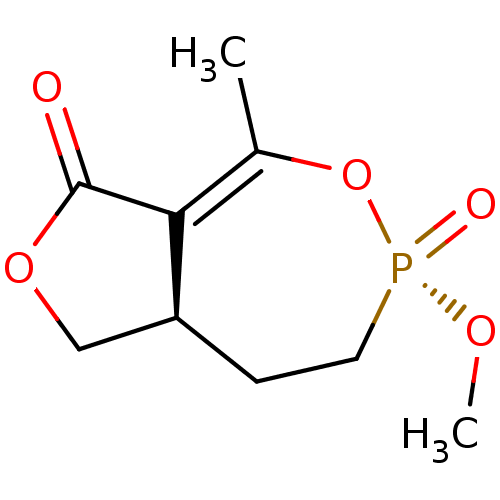 (CHEMBL592433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 30 min followed by substrate addition measured ever... | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241981 (CHEMBL4072457) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50241981 (CHEMBL4072457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50241979 (CHEMBL4066365) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241978 (CHEMBL4093928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241978 (CHEMBL4093928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of horse BChE using butyrylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50241980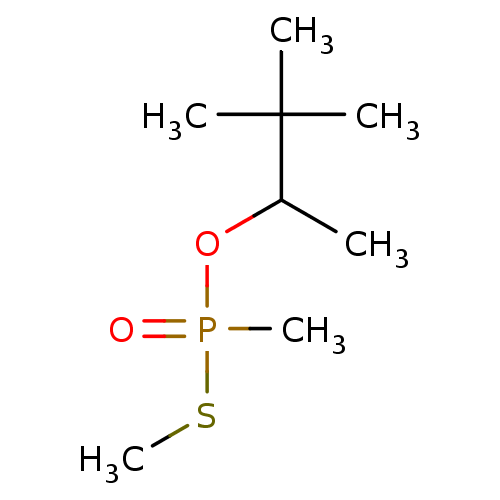 (CHEMBL4097266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50241978 (CHEMBL4093928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine iodide as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241980 (CHEMBL4097266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50241991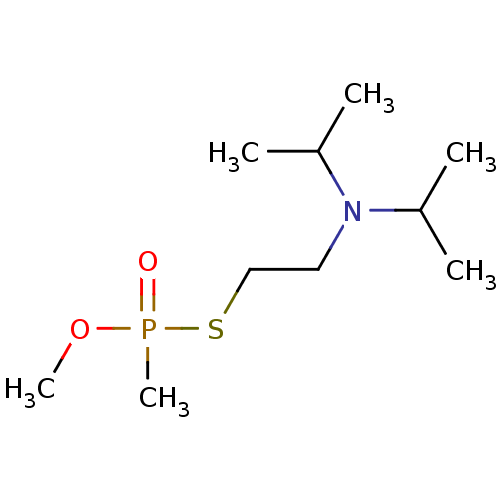 (CHEMBL4085438) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to Electric eel AChE | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50241982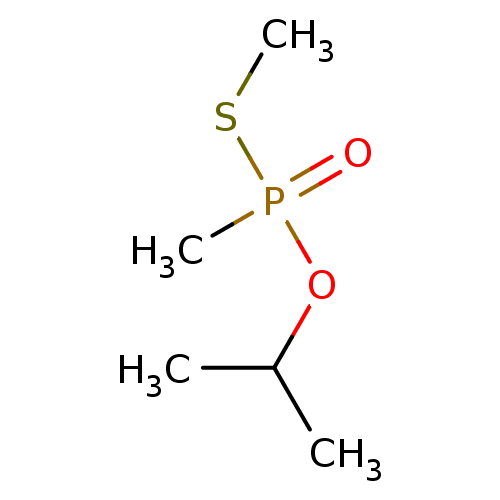 (CHEMBL4093201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.30E+6 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241987 (CHEMBL446997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to human AChE | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50027343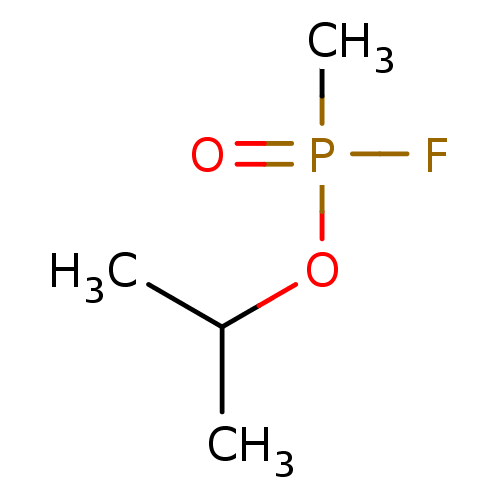 (CHEBI:75701 | ISOPROPYL METHYLPHOSPHONOFLUORIDATE) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to Electric eel AChE | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241982 (CHEMBL4093201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to human AChE using acetylthiocholine as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50240416 (CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity to Electric eel AChE using acetylthiocholine as substrate measured for 30 secs by Ellman's method | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292572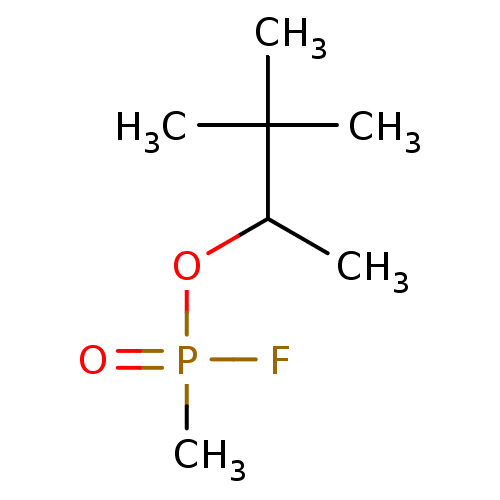 (3,3-dimethylbutan-2-yl methylphosphonofluoridate |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor from rat brain membranes using [3H]cyclohexyladenosine as radioligand. | Bioorg Med Chem 25: 3053-3058 (2017) Article DOI: 10.1016/j.bmc.2017.03.058 BindingDB Entry DOI: 10.7270/Q2H70J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||