

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50253249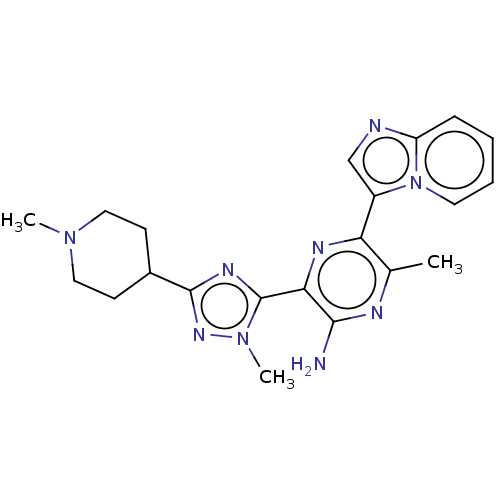 (CHEMBL4093351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50253251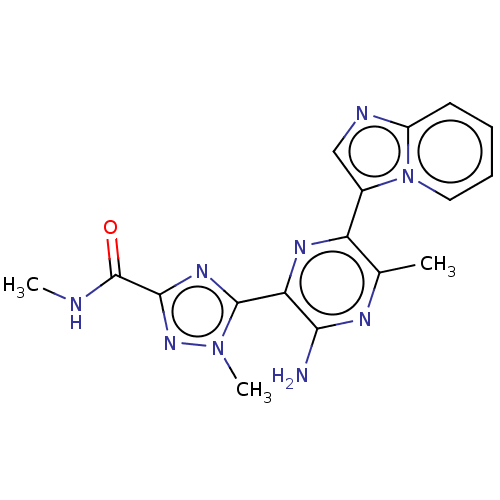 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50253250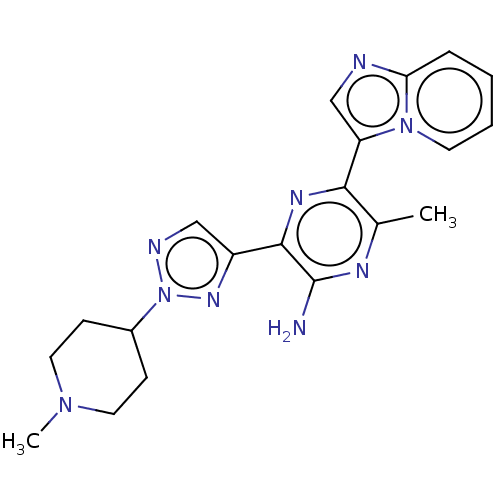 (CHEMBL4064039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50253243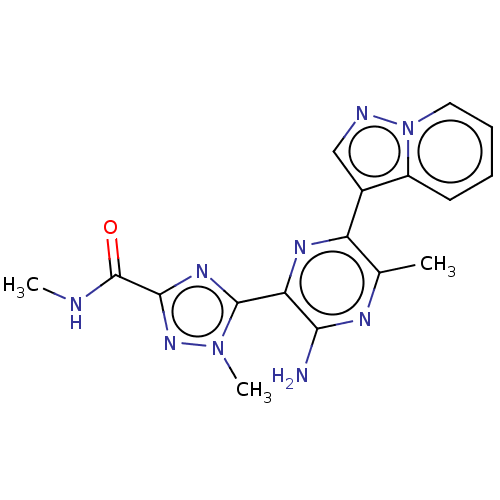 (CHEMBL4078681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50094473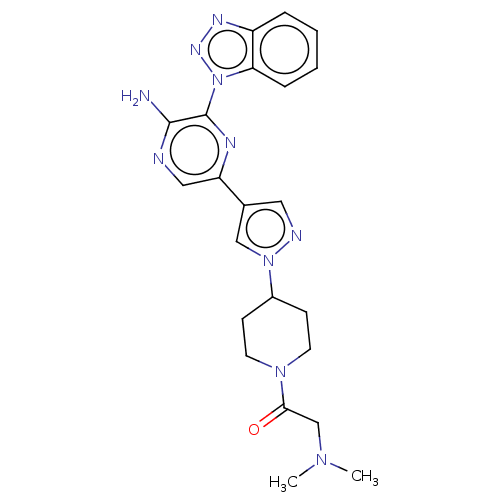 (CHEMBL3590219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50253248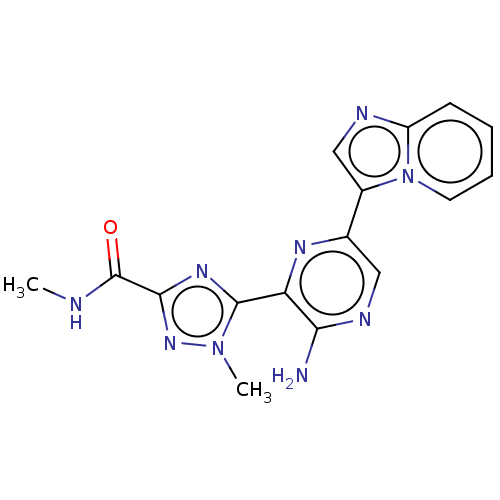 (CHEMBL4102957) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50094473 (CHEMBL3590219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253243 (CHEMBL4078681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253249 (CHEMBL4093351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253250 (CHEMBL4064039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253249 (CHEMBL4093351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50094473 (CHEMBL3590219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253250 (CHEMBL4064039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50094473 (CHEMBL3590219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253248 (CHEMBL4102957) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253253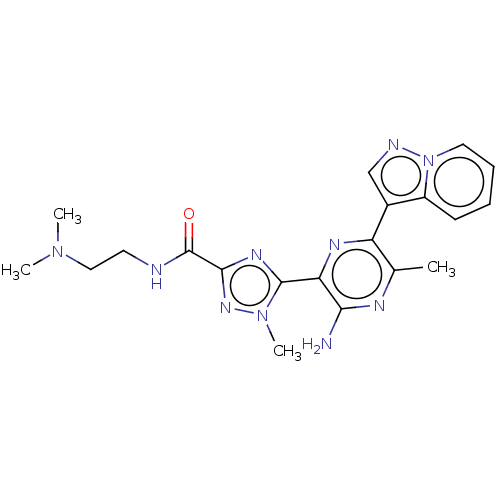 (CHEMBL4065083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253243 (CHEMBL4078681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50253251 (CHEMBL4072637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of human PI4K2alpha (1 to 479 residues) by ADP Glo HTS assay | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of human PI4KB (1 to 801 residues) by ADP Glo HTS assay | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50094473 (CHEMBL3590219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vacuolar protein sorting-associated protein 35 (Homo sapiens) | BDBM50253251 (CHEMBL4072637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of VPS35 (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253252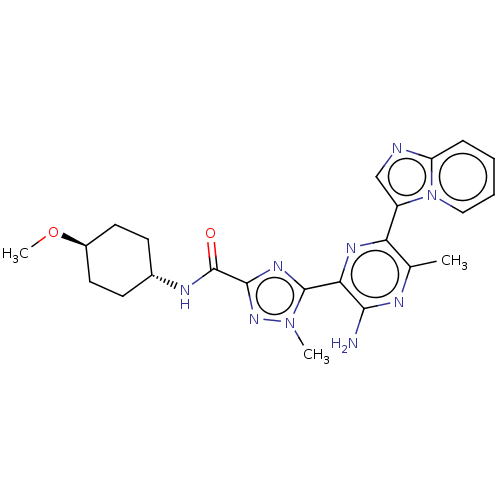 (CHEMBL4086526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoinositide 3-kinase regulatory subunit 4 (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of VPS15 (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50253248 (CHEMBL4102957) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as reduction in AKT phosphorylation at Ser473 | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50253251 (CHEMBL4072637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of m-TOR (unknown origin) | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50253251 (CHEMBL4072637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IMED Oncology, AstraZeneca, Darwin Building, Cambridge Science Park, 319 Milton Road, Cambridge CB4 0WG, United Kingdom. Electronic address: bernard.barlaam2@astrazeneca.com. Curated by ChEMBL | Assay Description Inhibition of human PIP5K2alpha (1 to 406 residues) by ADP Glo HTS assay | Bioorg Med Chem Lett 27: 3030-3035 (2017) Article DOI: 10.1016/j.bmcl.2017.05.028 BindingDB Entry DOI: 10.7270/Q23R0W9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||