

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | ||
| Ligand | BDBM50253252 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1685494 (CHEMBL4035973) | ||
| IC50 | 16000±n/a nM | ||
| Citation |  Barlaam, B; Cosulich, S; Fitzek, M; Germain, H; Green, S; Hanson, LL; Harris, CS; Hancox, U; Hudson, K; Lambert-van der Brempt, C; Lamorlette, M; Magnien, F; Ouvry, G; Page, K; Ruston, L; Ward, L; DelouvriÚ, B Discovery of a novel aminopyrazine series as selective PI3K? inhibitors. Bioorg Med Chem Lett27:3030-3035 (2017) [PubMed] Article Barlaam, B; Cosulich, S; Fitzek, M; Germain, H; Green, S; Hanson, LL; Harris, CS; Hancox, U; Hudson, K; Lambert-van der Brempt, C; Lamorlette, M; Magnien, F; Ouvry, G; Page, K; Ruston, L; Ward, L; DelouvriÚ, B Discovery of a novel aminopyrazine series as selective PI3K? inhibitors. Bioorg Med Chem Lett27:3030-3035 (2017) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | |||
| Name: | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | ||
| Synonyms: | PI3-kinase p110 subunit beta | PI3-kinase subunit p110-beta | PI3Kbeta | PIK3C1 | PIK3CB | PK3CB_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K-beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kÿ²) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | Phosphoinositide 3-Kinase (PI3K), beta | Phosphoinositide 3-Kinase (PI3K), beta Chain A | Phosphoinositide-3-kinase (PI3K beta) | PtdIns-3-kinase p110 | ||
| Type: | Enzyme Subunit | ||
| Mol. Mass.: | 122769.00 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P42338 | ||
| Residue: | 1070 | ||
| Sequence: |
| ||
| BDBM50253252 | |||
| n/a | |||
| Name | BDBM50253252 | ||
| Synonyms: | CHEMBL4086526 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H27N9O2 | ||
| Mol. Mass. | 461.5196 | ||
| SMILES | CO[C@H]1CC[C@@H](CC1)NC(=O)c1nc(-c2nc(-c3cnc4ccccn34)c(C)nc2N)n(C)n1 |r,wU:2.1,wD:5.8,(20.8,-29.62,;21.54,-28.27,;23.08,-28.24,;23.82,-26.89,;25.35,-26.85,;26.15,-28.17,;25.42,-29.52,;23.88,-29.56,;27.69,-28.13,;28.42,-26.77,;27.62,-25.46,;29.96,-26.73,;30.99,-27.88,;32.4,-27.25,;33.72,-28.02,;35.05,-27.25,;36.39,-28.01,;37.83,-27.49,;39.11,-28.35,;40.32,-27.41,;39.8,-25.97,;40.52,-24.62,;39.72,-23.31,;38.17,-23.36,;37.45,-24.71,;38.26,-26.01,;36.39,-29.56,;37.73,-30.33,;35.06,-30.33,;33.72,-29.56,;32.39,-30.33,;32.24,-25.72,;33.38,-24.69,;30.73,-25.4,)| | ||
| Structure | 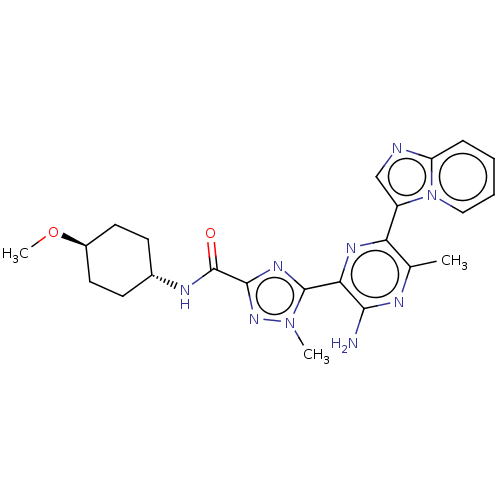
| ||