

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM27497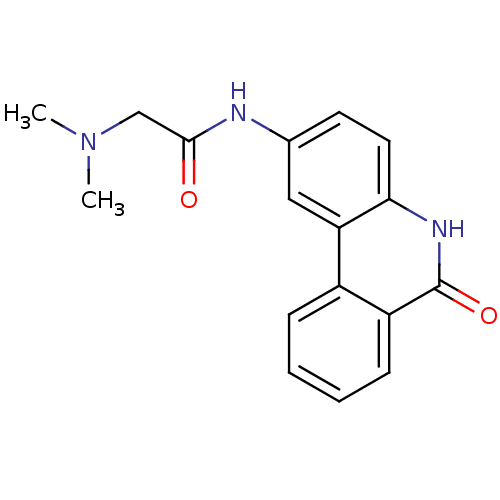 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-hydroxyphenylpyruvate dioxygenase from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Apis mellifera) | BDBM50242184 (Scalaradial) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-Hydroxyphenylpyruvate dioxygenase (HPPD) from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM50242184 (Scalaradial) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged TRPM2 expressed in HEK293 cells assessed as reduction in ADPR-induced intracellular calcium flux after 30 to 60 mins ... | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Rattus norvegicus) | BDBM50242184 (Scalaradial) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Inhibition of TRPM2 in rat INS-1 cells assessed as reduction in ADPR-induced intracellular calcium flux measured after 60 mins by whole cell patch cl... | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM27502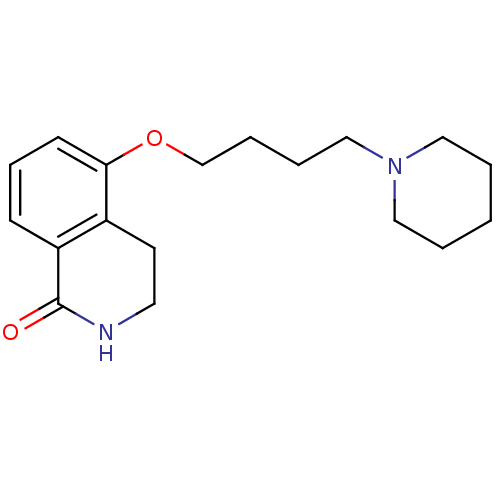 (3,4-dihydro-5-(4-(1-piperidinyl)butoxy)-1(2H)-isoq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-hydroxyphenylpyruvate dioxygenase from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 7 (Mus musculus) | BDBM50242184 (Scalaradial) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-Hydroxyphenylpyruvate dioxygenase (HPPD) from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM50278349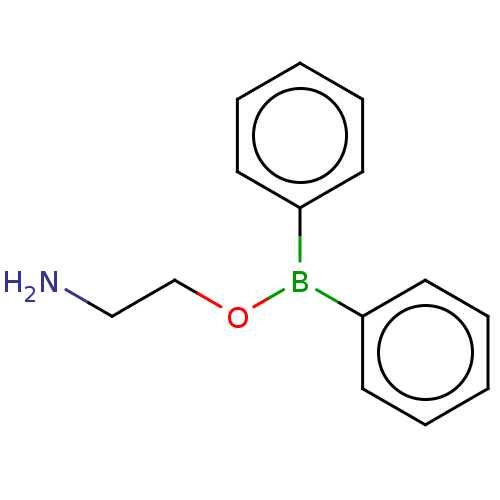 (2-(Diphenylboryloxy)Ethanamine | 2-Aminoethoxydiph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-Hydroxyphenylpyruvate dioxygenase (HPPD) from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM50227083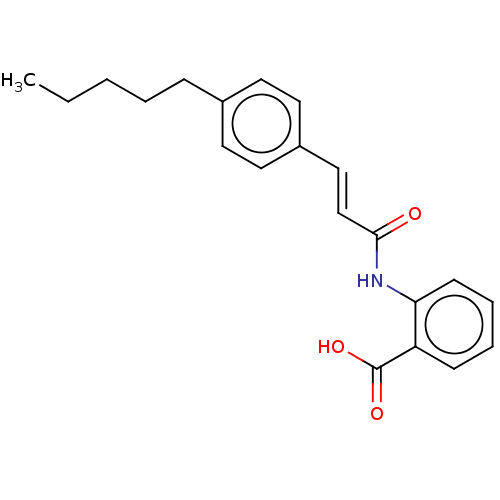 (CHEMBL173443) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-Hydroxyphenylpyruvate dioxygenase (HPPD) from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||