

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530476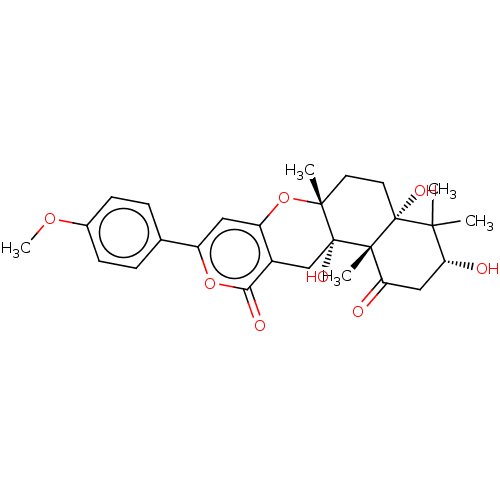 (CHEMBL4440372) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530476 (CHEMBL4440372) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50130210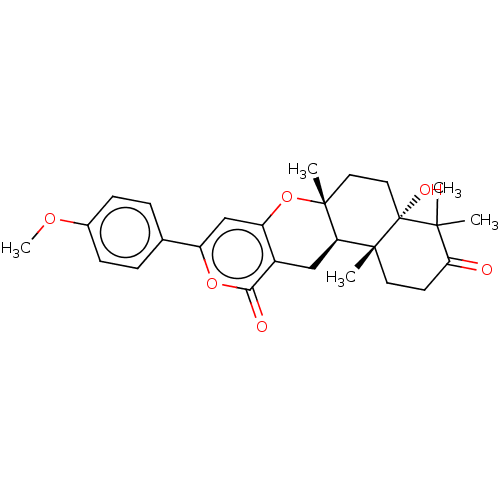 (CHEMBL3632852) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50130210 (CHEMBL3632852) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530478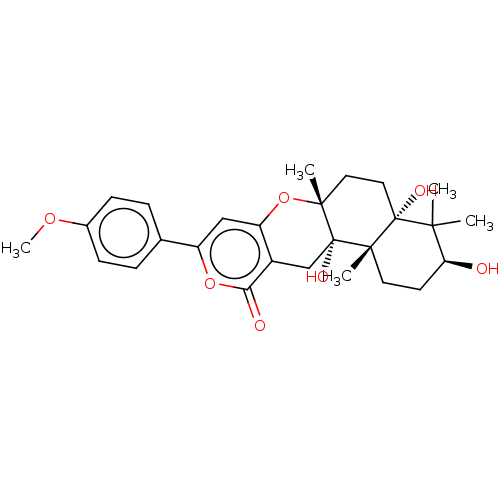 (CHEMBL4471625) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530478 (CHEMBL4471625) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530475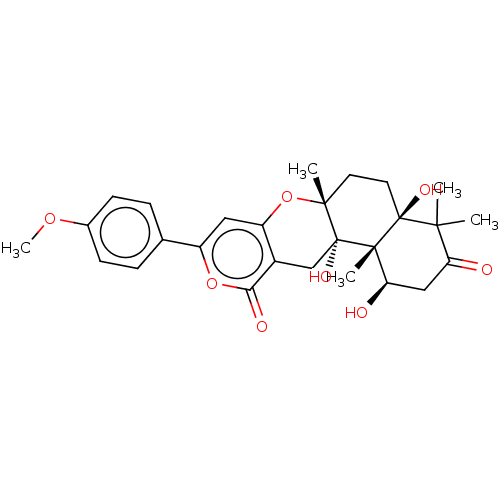 (CHEMBL4575080) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530475 (CHEMBL4575080) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530477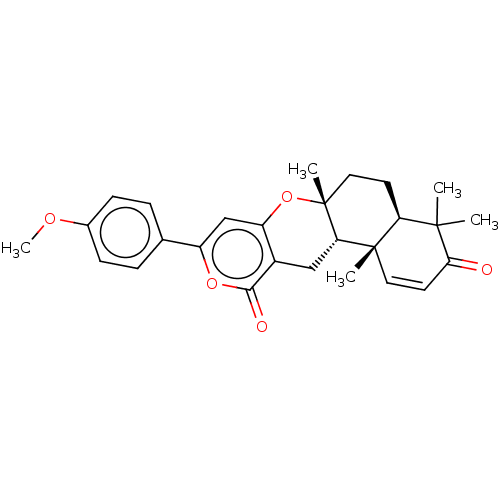 (CHEMBL4514571) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530477 (CHEMBL4514571) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10625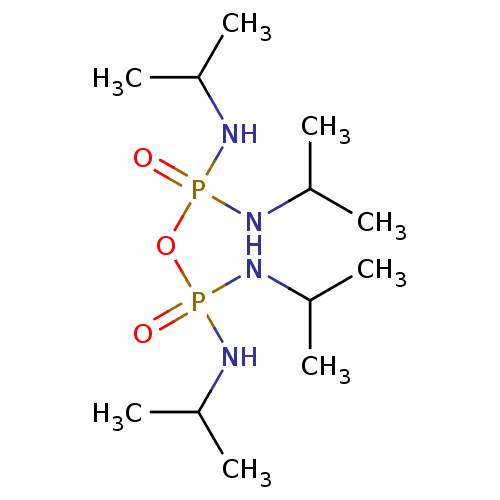 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530474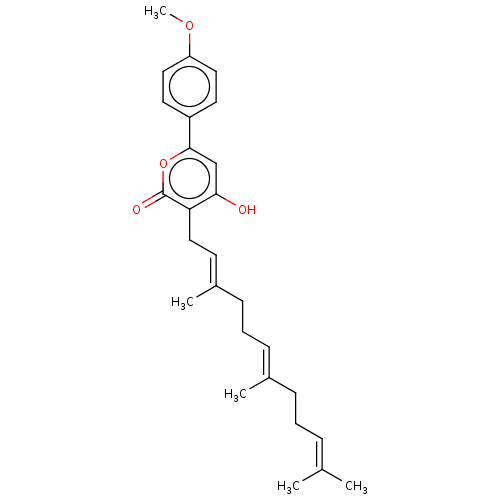 (CHEMBL4533666) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10625 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530479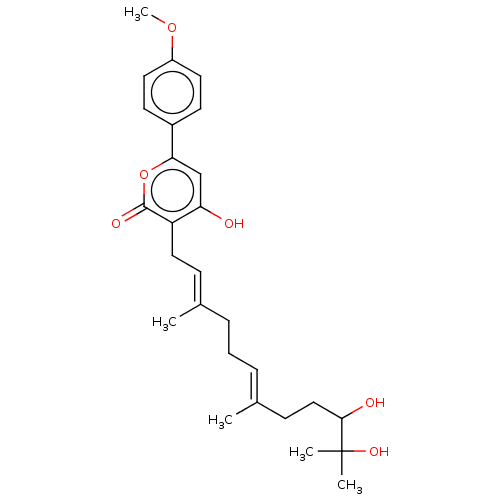 (CHEMBL4553044) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50130206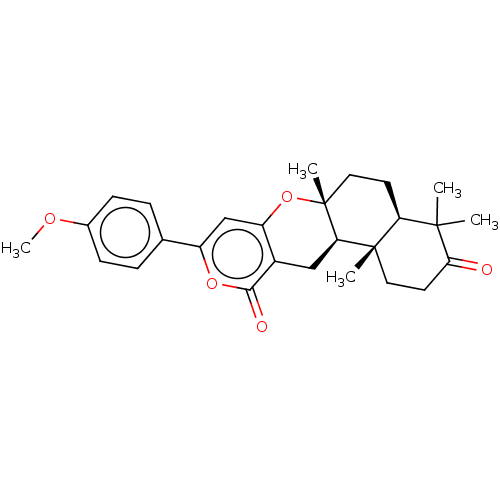 (CHEMBL3632856) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530473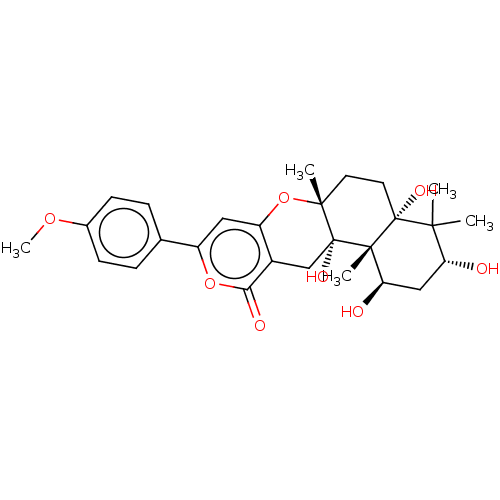 (CHEMBL4471396) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530474 (CHEMBL4533666) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530473 (CHEMBL4471396) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50530479 (CHEMBL4553044) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50130206 (CHEMBL3632856) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||