 Found 40 hits of Enzyme Inhibition Constant Data
Found 40 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282720

(CHEMBL288458 | methyl (2S)-2-{[(2S)-2-amino-1-cyan...)Show SMILES COC(=O)[C@H](CC(C)C)NC(C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C17H25N3O2/c1-12(2)9-15(17(21)22-3)20-16(11-18)14(19)10-13-7-5-4-6-8-13/h4-8,12,14-16,20H,9-10,19H2,1-3H3/t14-,15-,16?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282726
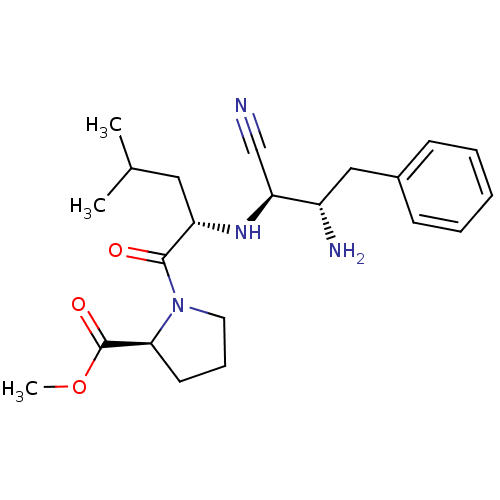
((S)-1-[(S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-pro...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)N[C@@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C22H32N4O3/c1-15(2)12-18(21(27)26-11-7-10-20(26)22(28)29-3)25-19(14-23)17(24)13-16-8-5-4-6-9-16/h4-6,8-9,15,17-20,25H,7,10-13,24H2,1-3H3/t17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282715
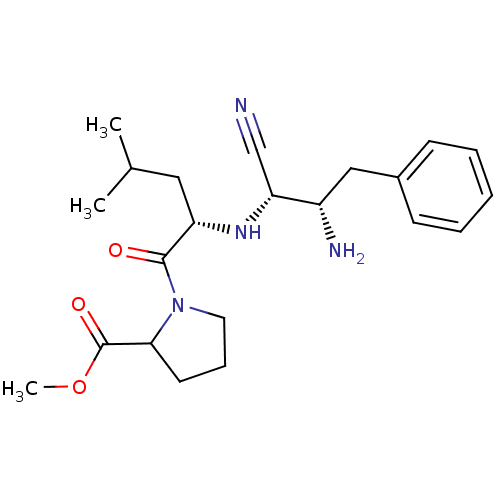
(1-[(S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-propyla...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C22H32N4O3/c1-15(2)12-18(21(27)26-11-7-10-20(26)22(28)29-3)25-19(14-23)17(24)13-16-8-5-4-6-9-16/h4-6,8-9,15,17-20,25H,7,10-13,24H2,1-3H3/t17-,18-,19+,20?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282725

(CHEMBL35627 | methyl (2S)-2-{[(2S)-2-amino-1-cyano...)Show SMILES COC(=O)[C@@H](NC(C#N)[C@@H](N)Cc1ccccc1)C(C)C Show InChI InChI=1S/C16H23N3O2/c1-11(2)15(16(20)21-3)19-14(10-17)13(18)9-12-7-5-4-6-8-12/h4-8,11,13-15,19H,9,18H2,1-3H3/t13-,14?,15-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282728
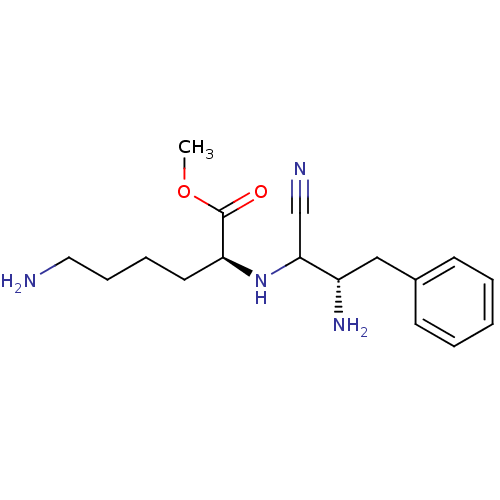
(CHEMBL36081 | methyl (2S)-6-amino-2-{[(2S)-2-amino...)Show SMILES COC(=O)[C@H](CCCCN)NC(C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C17H26N4O2/c1-23-17(22)15(9-5-6-10-18)21-16(12-19)14(20)11-13-7-3-2-4-8-13/h2-4,7-8,14-16,21H,5-6,9-11,18,20H2,1H3/t14-,15-,16?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282715

(1-[(S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-propyla...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C22H32N4O3/c1-15(2)12-18(21(27)26-11-7-10-20(26)22(28)29-3)25-19(14-23)17(24)13-16-8-5-4-6-9-16/h4-6,8-9,15,17-20,25H,7,10-13,24H2,1-3H3/t17-,18-,19+,20?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aminopeptidase B
(Mus musculus) | BDBM50282726

((S)-1-[(S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-pro...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)N[C@@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C22H32N4O3/c1-15(2)12-18(21(27)26-11-7-10-20(26)22(28)29-3)25-19(14-23)17(24)13-16-8-5-4-6-9-16/h4-6,8-9,15,17-20,25H,7,10-13,24H2,1-3H3/t17-,18-,19-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282725

(CHEMBL35627 | methyl (2S)-2-{[(2S)-2-amino-1-cyano...)Show SMILES COC(=O)[C@@H](NC(C#N)[C@@H](N)Cc1ccccc1)C(C)C Show InChI InChI=1S/C16H23N3O2/c1-11(2)15(16(20)21-3)19-14(10-17)13(18)9-12-7-5-4-6-8-12/h4-8,11,13-15,19H,9,18H2,1-3H3/t13-,14?,15-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282728

(CHEMBL36081 | methyl (2S)-6-amino-2-{[(2S)-2-amino...)Show SMILES COC(=O)[C@H](CCCCN)NC(C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C17H26N4O2/c1-23-17(22)15(9-5-6-10-18)21-16(12-19)14(20)11-13-7-3-2-4-8-13/h2-4,7-8,14-16,21H,5-6,9-11,18,20H2,1H3/t14-,15-,16?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282720

(CHEMBL288458 | methyl (2S)-2-{[(2S)-2-amino-1-cyan...)Show SMILES COC(=O)[C@H](CC(C)C)NC(C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C17H25N3O2/c1-12(2)9-15(17(21)22-3)20-16(11-18)14(19)10-13-7-5-4-6-8-13/h4-8,12,14-16,20H,9-10,19H2,1-3H3/t14-,15-,16?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282722

((S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-propylamin...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C20H23N3O2/c1-25-20(24)18(13-16-10-6-3-7-11-16)23-19(14-21)17(22)12-15-8-4-2-5-9-15/h2-11,17-19,23H,12-13,22H2,1H3/t17-,18-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282729
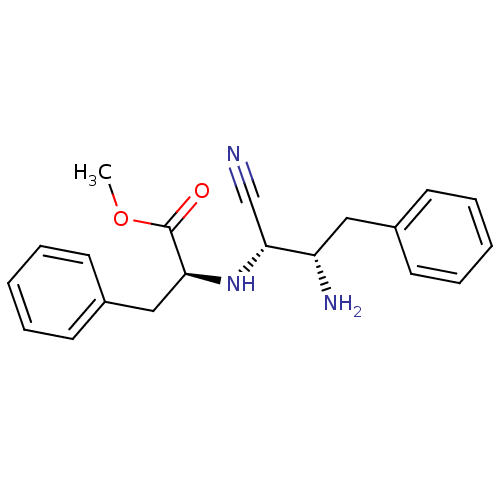
((S)-2-((1S,2S)-2-Amino-1-cyano-3-phenyl-propylamin...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C20H23N3O2/c1-25-20(24)18(13-16-10-6-3-7-11-16)23-19(14-21)17(22)12-15-8-4-2-5-9-15/h2-11,17-19,23H,12-13,22H2,1H3/t17-,18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282722

((S)-2-((1R,2S)-2-Amino-1-cyano-3-phenyl-propylamin...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C20H23N3O2/c1-25-20(24)18(13-16-10-6-3-7-11-16)23-19(14-21)17(22)12-15-8-4-2-5-9-15/h2-11,17-19,23H,12-13,22H2,1H3/t17-,18-,19-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282729

((S)-2-((1S,2S)-2-Amino-1-cyano-3-phenyl-propylamin...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@H](C#N)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C20H23N3O2/c1-25-20(24)18(13-16-10-6-3-7-11-16)23-19(14-21)17(22)12-15-8-4-2-5-9-15/h2-11,17-19,23H,12-13,22H2,1H3/t17-,18-,19+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282718

(1-[(S)-2-((1S,2S)-2-Amino-1-carbamoyl-3-phenyl-pro...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C22H34N4O4/c1-14(2)12-17(21(28)26-11-7-10-18(26)22(29)30-3)25-19(20(24)27)16(23)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-19,25H,7,10-13,23H2,1-3H3,(H2,24,27)/t16-,17-,18?,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory potency on Aminopeptidase M using L-leucine-2-naphthylamide hydrochloride as substrate(0.5 mM, Km=0.6*10e-4 M) |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282717

(1-[(S)-2-((S)-2-Amino-3-phenyl-propionylamino)-4-m...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C21H31N3O4/c1-14(2)12-17(20(26)24-11-7-10-18(24)21(27)28-3)23-19(25)16(22)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-18H,7,10-13,22H2,1-3H3,(H,23,25)/t16-,17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aminopeptidase B
(Mus musculus) | BDBM50282717

(1-[(S)-2-((S)-2-Amino-3-phenyl-propionylamino)-4-m...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C21H31N3O4/c1-14(2)12-17(20(26)24-11-7-10-18(24)21(27)28-3)23-19(25)16(22)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-18H,7,10-13,22H2,1-3H3,(H,23,25)/t16-,17-,18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282712
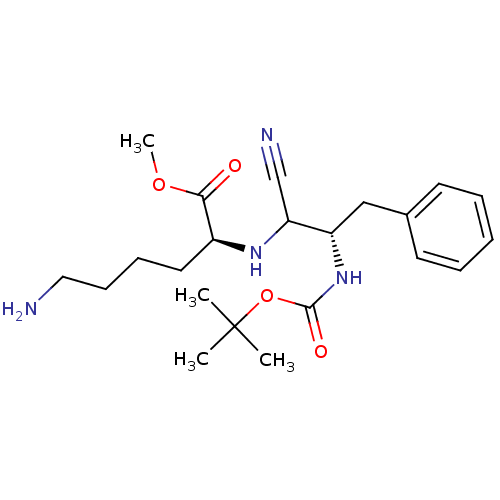
((S)-6-Amino-2-((S)-2-tert-butoxycarbonylamino-1-cy...)Show SMILES COC(=O)[C@H](CCCCN)NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C22H34N4O4/c1-22(2,3)30-21(28)26-18(14-16-10-6-5-7-11-16)19(15-24)25-17(20(27)29-4)12-8-9-13-23/h5-7,10-11,17-19,25H,8-9,12-14,23H2,1-4H3,(H,26,28)/t17-,18-,19?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50027516

(2-(2-Amino-3-phenyl-propionylamino)-4-methyl-penta...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H22N2O3/c1-10(2)8-13(15(19)20)17-14(18)12(16)9-11-6-4-3-5-7-11/h3-7,10,12-13H,8-9,16H2,1-2H3,(H,17,18)(H,19,20)/t12-,13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282719
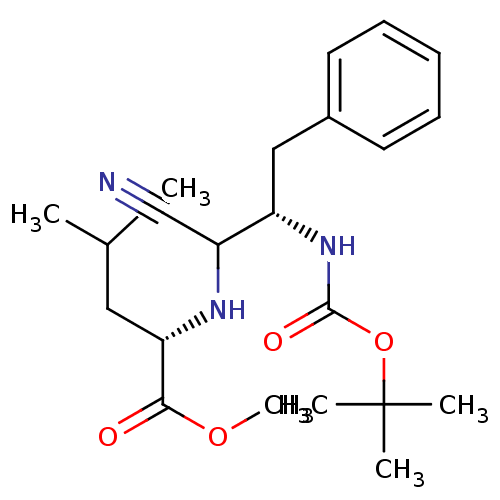
((S)-2-((S)-2-tert-Butoxycarbonylamino-1-cyano-3-ph...)Show SMILES COC(=O)[C@H](CC(C)C)NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C22H33N3O4/c1-15(2)12-18(20(26)28-6)24-19(14-23)17(13-16-10-8-7-9-11-16)25-21(27)29-22(3,4)5/h7-11,15,17-19,24H,12-13H2,1-6H3,(H,25,27)/t17-,18-,19?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282713

((S)-2-((S)-2-tert-Butoxycarbonylamino-1-cyano-3-ph...)Show SMILES COC(=O)[C@@H](NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C21H31N3O4/c1-14(2)18(19(25)27-6)23-17(13-22)16(12-15-10-8-7-9-11-15)24-20(26)28-21(3,4)5/h7-11,14,16-18,23H,12H2,1-6H3,(H,24,26)/t16-,17?,18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282721

(1-[(S)-2-((1S,2S)-2-tert-Butoxycarbonylamino-1-cya...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C27H40N4O5/c1-18(2)15-21(24(32)31-14-10-13-23(31)25(33)35-6)29-22(17-28)20(16-19-11-8-7-9-12-19)30-26(34)36-27(3,4)5/h7-9,11-12,18,20-23,29H,10,13-16H2,1-6H3,(H,30,34)/t20-,21-,22-,23?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50027516

(2-(2-Amino-3-phenyl-propionylamino)-4-methyl-penta...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H22N2O3/c1-10(2)8-13(15(19)20)17-14(18)12(16)9-11-6-4-3-5-7-11/h3-7,10,12-13H,8-9,16H2,1-2H3,(H,17,18)(H,19,20)/t12-,13-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282714
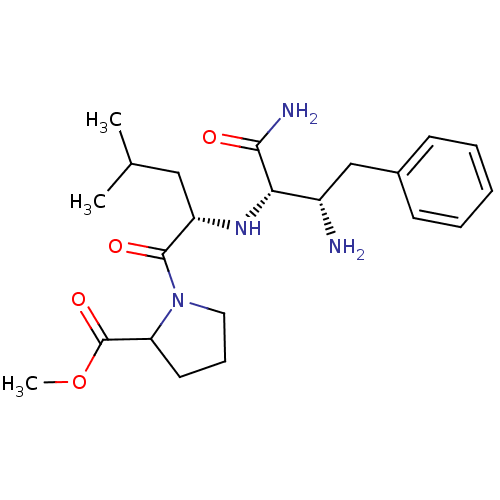
(1-[(S)-2-((1R,2S)-2-Amino-1-carbamoyl-3-phenyl-pro...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C22H34N4O4/c1-14(2)12-17(21(28)26-11-7-10-18(26)22(29)30-3)25-19(20(24)27)16(23)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-19,25H,7,10-13,23H2,1-3H3,(H2,24,27)/t16-,17-,18?,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282724
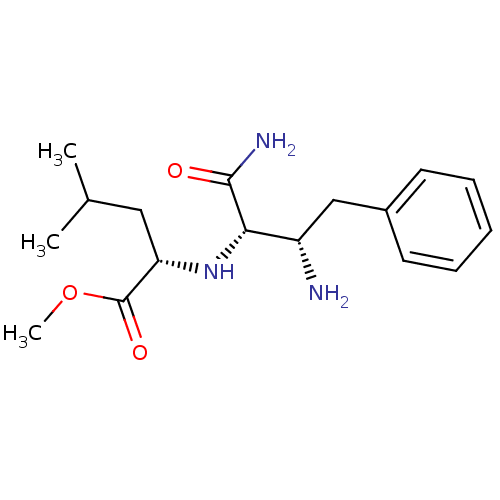
((S)-2-((1R,2S)-2-Amino-1-carbamoyl-3-phenyl-propyl...)Show SMILES COC(=O)[C@H](CC(C)C)N[C@@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C17H27N3O3/c1-11(2)9-14(17(22)23-3)20-15(16(19)21)13(18)10-12-7-5-4-6-8-12/h4-8,11,13-15,20H,9-10,18H2,1-3H3,(H2,19,21)/t13-,14-,15-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aminopeptidase B
(Mus musculus) | BDBM50282718

(1-[(S)-2-((1S,2S)-2-Amino-1-carbamoyl-3-phenyl-pro...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C22H34N4O4/c1-14(2)12-17(21(28)26-11-7-10-18(26)22(29)30-3)25-19(20(24)27)16(23)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-19,25H,7,10-13,23H2,1-3H3,(H2,24,27)/t16-,17-,18?,19+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory potency against Aminopeptidase B in murine L-cells by aoyagi method using L-lysine-2-naphthylamide hydrochlorid... |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282719

((S)-2-((S)-2-tert-Butoxycarbonylamino-1-cyano-3-ph...)Show SMILES COC(=O)[C@H](CC(C)C)NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C22H33N3O4/c1-15(2)12-18(20(26)28-6)24-19(14-23)17(13-16-10-8-7-9-11-16)25-21(27)29-22(3,4)5/h7-11,15,17-19,24H,12-13H2,1-6H3,(H,25,27)/t17-,18-,19?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282723
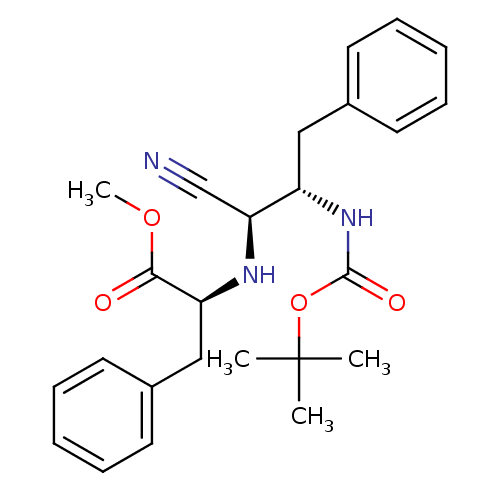
((S)-2-((1S,2S)-2-tert-Butoxycarbonylamino-1-cyano-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C25H31N3O4/c1-25(2,3)32-24(30)28-20(15-18-11-7-5-8-12-18)22(17-26)27-21(23(29)31-4)16-19-13-9-6-10-14-19/h5-14,20-22,27H,15-16H2,1-4H3,(H,28,30)/t20-,21-,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282714

(1-[(S)-2-((1R,2S)-2-Amino-1-carbamoyl-3-phenyl-pro...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C22H34N4O4/c1-14(2)12-17(21(28)26-11-7-10-18(26)22(29)30-3)25-19(20(24)27)16(23)13-15-8-5-4-6-9-15/h4-6,8-9,14,16-19,25H,7,10-13,23H2,1-3H3,(H2,24,27)/t16-,17-,18?,19-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282711
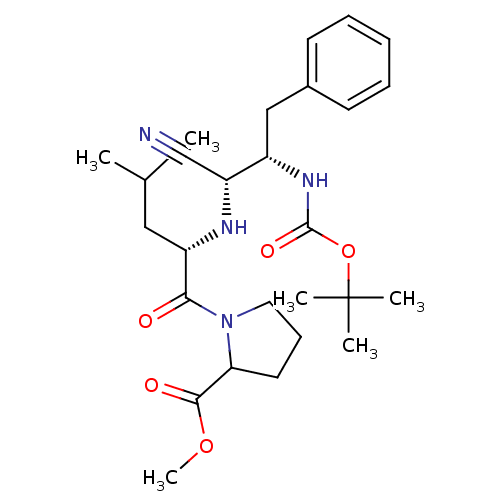
(1-[(S)-2-((1R,2S)-2-tert-Butoxycarbonylamino-1-cya...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C27H40N4O5/c1-18(2)15-21(24(32)31-14-10-13-23(31)25(33)35-6)29-22(17-28)20(16-19-11-8-7-9-12-19)30-26(34)36-27(3,4)5/h7-9,11-12,18,20-23,29H,10,13-16H2,1-6H3,(H,30,34)/t20-,21-,22+,23?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282716

((S)-2-((1R,2S)-2-tert-Butoxycarbonylamino-1-cyano-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C25H31N3O4/c1-25(2,3)32-24(30)28-20(15-18-11-7-5-8-12-18)22(17-26)27-21(23(29)31-4)16-19-13-9-6-10-14-19/h5-14,20-22,27H,15-16H2,1-4H3,(H,28,30)/t20-,21-,22+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282716

((S)-2-((1R,2S)-2-tert-Butoxycarbonylamino-1-cyano-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C25H31N3O4/c1-25(2,3)32-24(30)28-20(15-18-11-7-5-8-12-18)22(17-26)27-21(23(29)31-4)16-19-13-9-6-10-14-19/h5-14,20-22,27H,15-16H2,1-4H3,(H,28,30)/t20-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282723

((S)-2-((1S,2S)-2-tert-Butoxycarbonylamino-1-cyano-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)N[C@@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C25H31N3O4/c1-25(2,3)32-24(30)28-20(15-18-11-7-5-8-12-18)22(17-26)27-21(23(29)31-4)16-19-13-9-6-10-14-19/h5-14,20-22,27H,15-16H2,1-4H3,(H,28,30)/t20-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50282724

((S)-2-((1R,2S)-2-Amino-1-carbamoyl-3-phenyl-propyl...)Show SMILES COC(=O)[C@H](CC(C)C)N[C@@H]([C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C17H27N3O3/c1-11(2)9-14(17(22)23-3)20-15(16(19)21)13(18)10-12-7-5-4-6-8-12/h4-8,11,13-15,20H,9-10,18H2,1-3H3,(H2,19,21)/t13-,14-,15-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase B in murine L-cells by Aoyagi method. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282721

(1-[(S)-2-((1S,2S)-2-tert-Butoxycarbonylamino-1-cya...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C27H40N4O5/c1-18(2)15-21(24(32)31-14-10-13-23(31)25(33)35-6)29-22(17-28)20(16-19-11-8-7-9-12-19)30-26(34)36-27(3,4)5/h7-9,11-12,18,20-23,29H,10,13-16H2,1-6H3,(H,30,34)/t20-,21-,22-,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282711

(1-[(S)-2-((1R,2S)-2-tert-Butoxycarbonylamino-1-cya...)Show SMILES COC(=O)C1CCCN1C(=O)[C@H](CC(C)C)N[C@H](C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C27H40N4O5/c1-18(2)15-21(24(32)31-14-10-13-23(31)25(33)35-6)29-22(17-28)20(16-19-11-8-7-9-12-19)30-26(34)36-27(3,4)5/h7-9,11-12,18,20-23,29H,10,13-16H2,1-6H3,(H,30,34)/t20-,21-,22+,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282712

((S)-6-Amino-2-((S)-2-tert-butoxycarbonylamino-1-cy...)Show SMILES COC(=O)[C@H](CCCCN)NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C22H34N4O4/c1-22(2,3)30-21(28)26-18(14-16-10-6-5-7-11-16)19(15-24)25-17(20(27)29-4)12-8-9-13-23/h5-7,10-11,17-19,25H,8-9,12-14,23H2,1-4H3,(H,26,28)/t17-,18-,19?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50282713

((S)-2-((S)-2-tert-Butoxycarbonylamino-1-cyano-3-ph...)Show SMILES COC(=O)[C@@H](NC(C#N)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C21H31N3O4/c1-14(2)18(19(25)27-6)23-17(13-22)16(12-15-10-8-7-9-11-15)24-20(26)28-21(3,4)5/h7-11,14,16-18,23H,12H2,1-6H3,(H,24,26)/t16-,17?,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Aminopeptidase M. |
Bioorg Med Chem Lett 4: 1491-1496 (1994)
Article DOI: 10.1016/S0960-894X(01)80519-9
BindingDB Entry DOI: 10.7270/Q26M37B6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data