

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50029588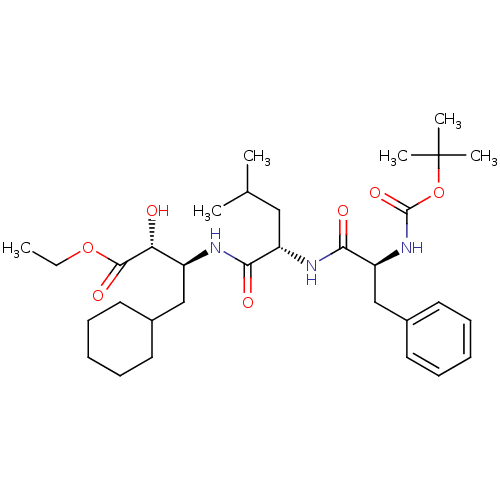 ((2R,3S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029584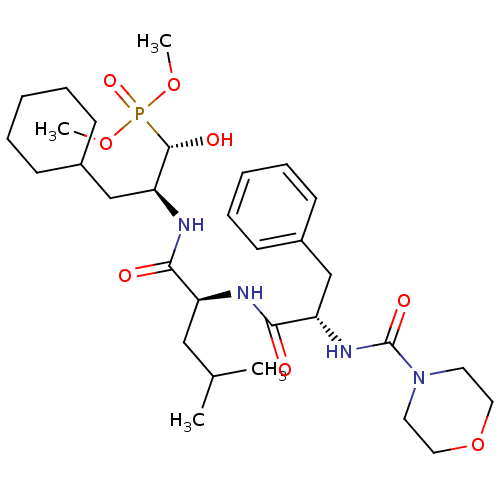 (CHEMBL334907 | [(1S,2S)-3-Cyclohexyl-1-hydroxy-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029580 (CHEMBL344155 | {(1S,2S)-2-[(S)-2-((S)-2-tert-Butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029589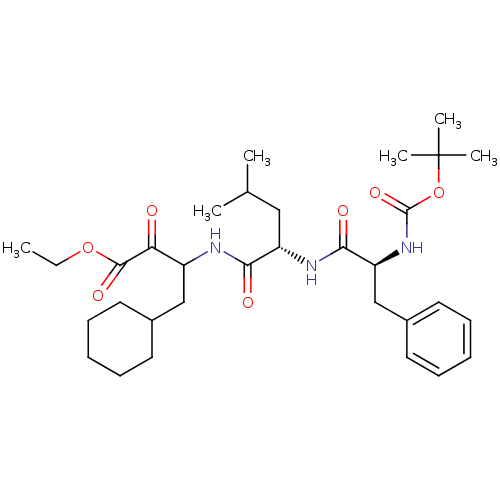 (3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029591 (((1S,2S)-3-Cyclohexyl-2-{(S)-2-[(S)-2-(cyclopentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029586 (CHEMBL407513 | {(1S,2S)-2-[(S)-2-((S)-2-tert-Butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029592 (((1S,2S)-2-{(S)-2-[(S)-2-(6-Amino-hexanoylamino)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029578 (((1S,2S)-3-Cyclohexyl-2-{(S)-2-[(S)-2-(cyclopentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029582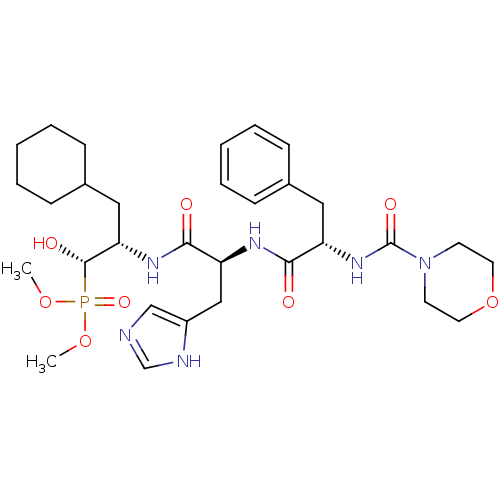 (CHEMBL336245 | [(1S,2S)-3-Cyclohexyl-1-hydroxy-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029581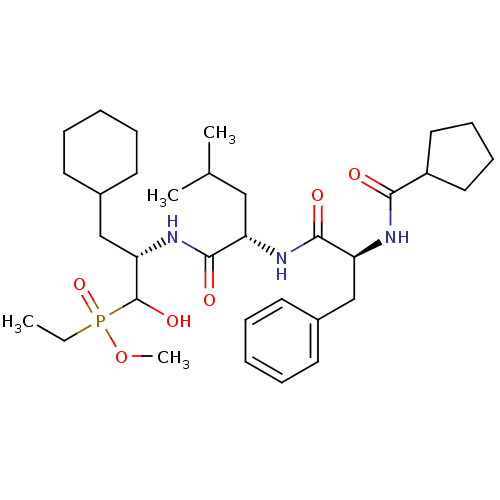 (((S)-3-Cyclohexyl-2-{(S)-2-[(S)-2-(cyclopentanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. value in parentheses indicate no. of determinations | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029581 (((S)-3-Cyclohexyl-2-{(S)-2-[(S)-2-(cyclopentanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. value in parentheses indicate no. of determinations | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029579 (CHEMBL436018 | {(1S,2S)-3-Cyclohexyl-2-[(S)-2-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029590 (CHEMBL140422 | Cyclopentanecarboxylic acid ((S)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. value in parentheses indicate no. of determinations | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029583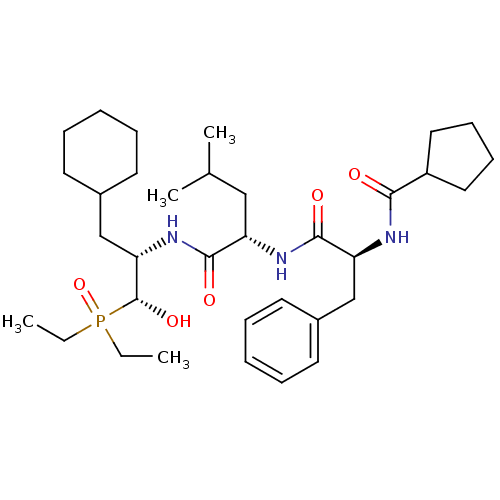 (CHEMBL343814 | Cyclopentanecarboxylic acid ((S)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. value in parentheses indicate no. of determinations | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029587 (((1S,2S)-3-Cyclohexyl-2-{(S)-2-[(S)-2-(cyclopentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50029585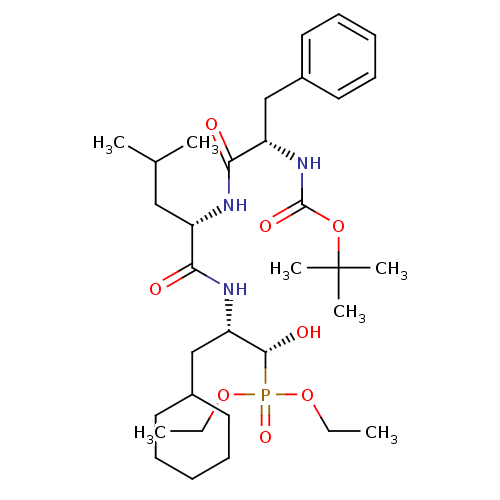 (CHEMBL140146 | {(1R,2S)-2-[(S)-2-((S)-2-tert-Butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Evaluation of inhibitory activity of the compound against human renin. | J Med Chem 38: 4557-69 (1995) BindingDB Entry DOI: 10.7270/Q27D2T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||