

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4007 (1 H-indole-3-alkanamide deriv. 10p | 2,2 -Dithiobi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4008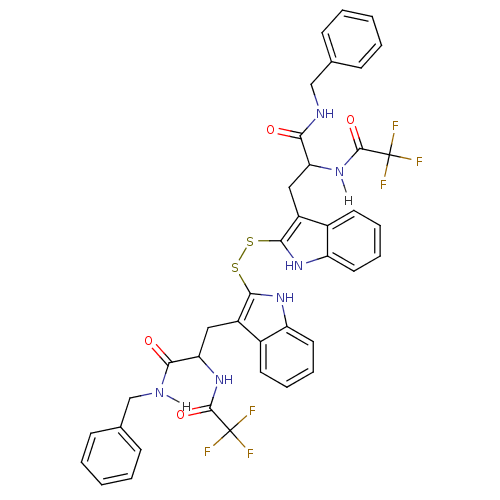 (1 H-indole-3-alkanamide deriv. 10q | 2,2-Dithiobis...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3997 (1 H-indole-3-alkanamide deriv. 10f | N-methyl-3-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4001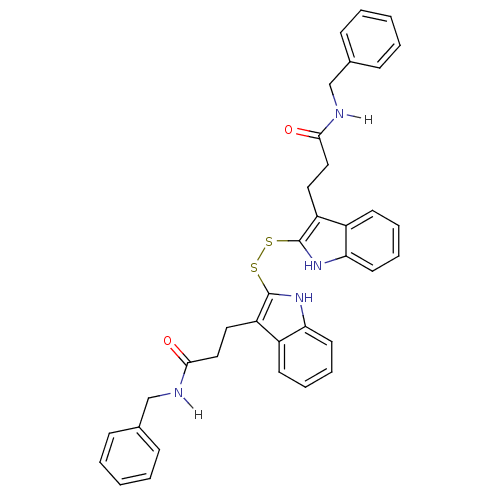 (1 H-indole-3-alkanamide deriv. 10j | N-benzyl-3-[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3999 (1 H-indole-3-alkanamide deriv. 10h | 3-[2-({3-[2-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4012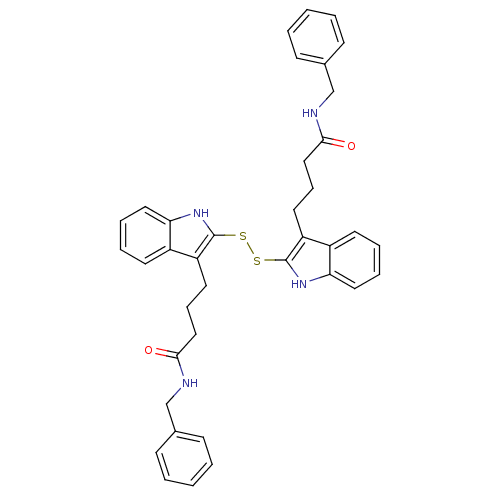 (1 H-indole-3-alkanamide deriv. 10u | N-benzyl-4-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3996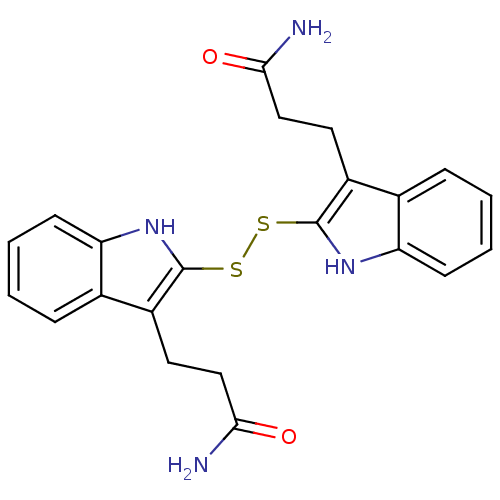 (1 H-indole-3-alkanamide deriv. 10e | 2,2 -Dithiobi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3351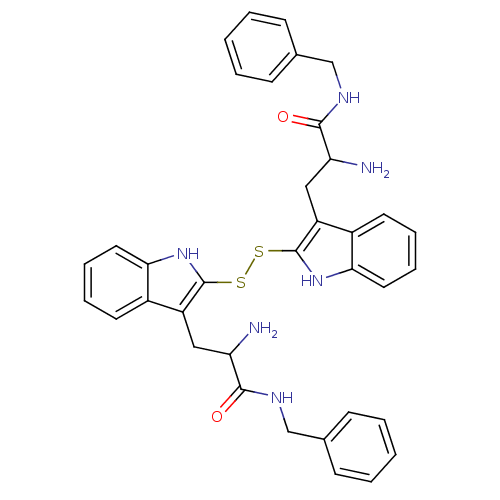 (2-amino-3-[2-({3-[2-amino-2-(benzylcarbamoyl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4003 (1 H-indole-3-alkanamide deriv. 10l | 2,2 -Dithiobi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4002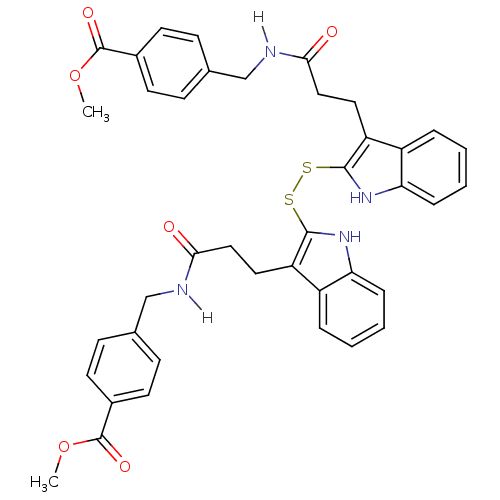 (1 H-indole-3-alkanamide deriv. 10k | 2,2 -dithiobi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4014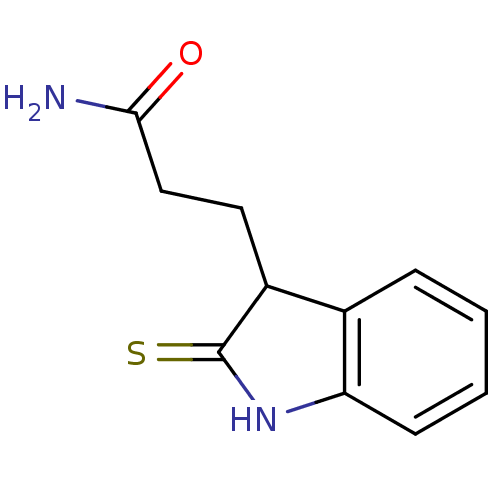 (1 H-indole-3-alkanamide deriv. 11e | 2,3-Dihydro-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3991 (1 H-indole-3-alkanamide deriv. 10b | 2-(2-{[3-(cya...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3998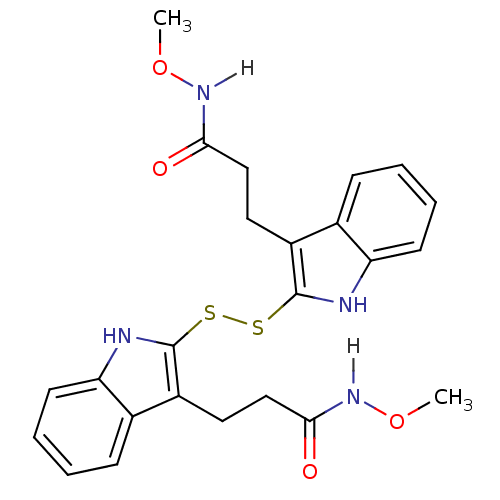 (1 H-indole-3-alkanamide deriv. 10g | N-methoxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4001 (1 H-indole-3-alkanamide deriv. 10j | N-benzyl-3-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4013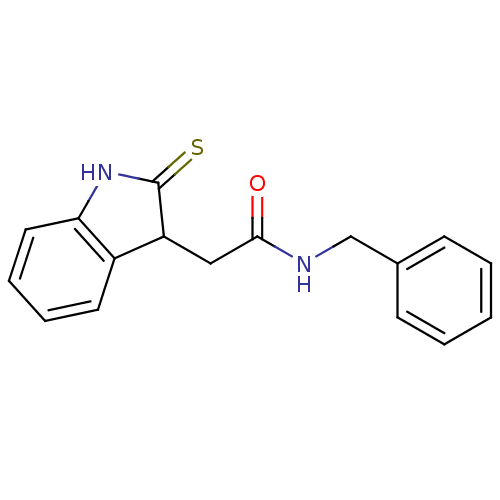 (1 H-indole-3-alkanamide deriv. 11a | N-benzyl-2-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3981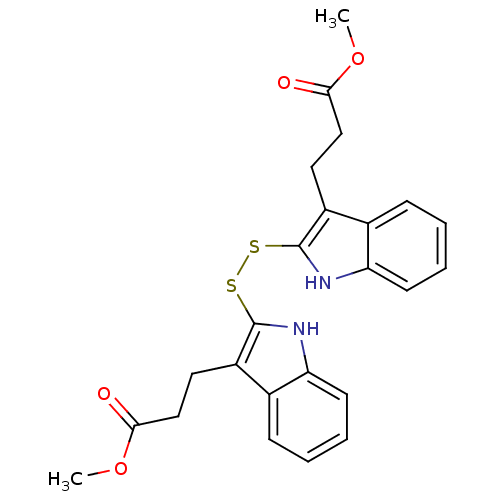 (1 H-indole-3-alkanamide deriv. 7 | Dimethyl 2,2 -D...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3990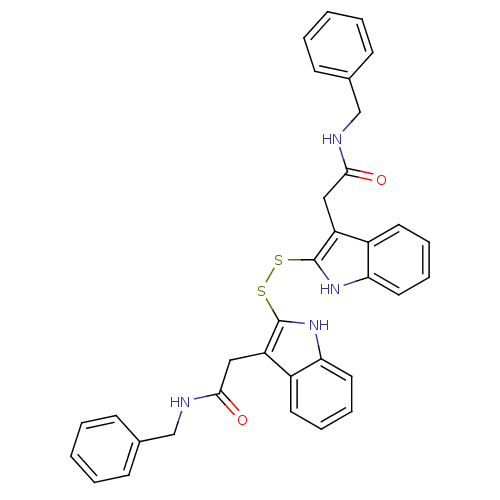 (1 H-indole-3-alkanamide deriv. 10a | N-benzyl-2-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4000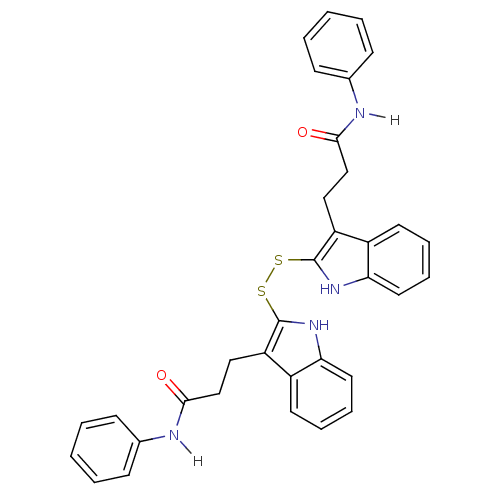 (1 H-indole-3-alkanamide deriv. 10i | 2,2-Dithiobis...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3979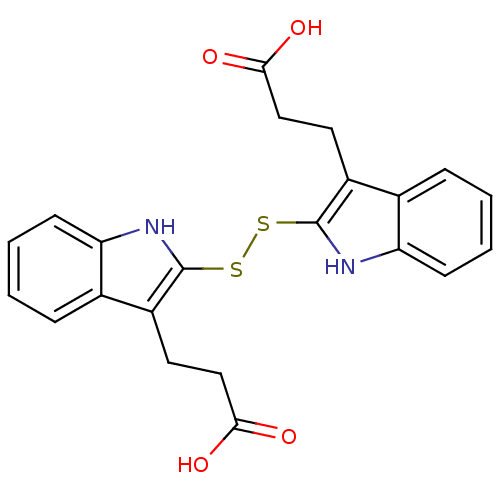 (2,2 -Dithiobis(1H-indole-3-propanoic acid) | 3-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4003 (1 H-indole-3-alkanamide deriv. 10l | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3351 (2-amino-3-[2-({3-[2-amino-2-(benzylcarbamoyl)ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4005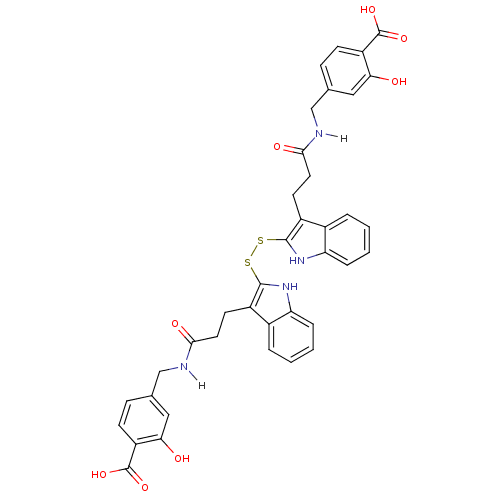 (1 H-indole-3-alkanamide deriv. 10n | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4015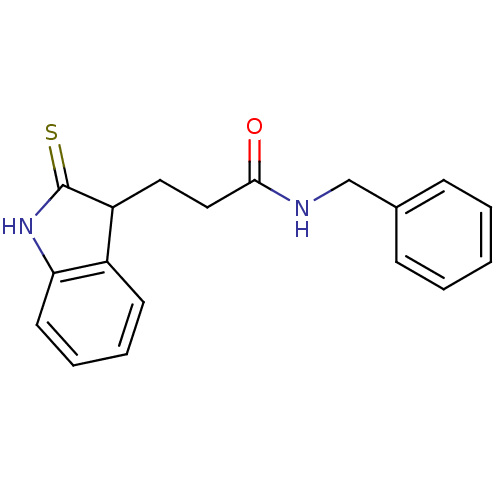 (1 H-indole-3-alkanamide deriv. 11j | N-benzyl-3-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3997 (1 H-indole-3-alkanamide deriv. 10f | N-methyl-3-[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3999 (1 H-indole-3-alkanamide deriv. 10h | 3-[2-({3-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4006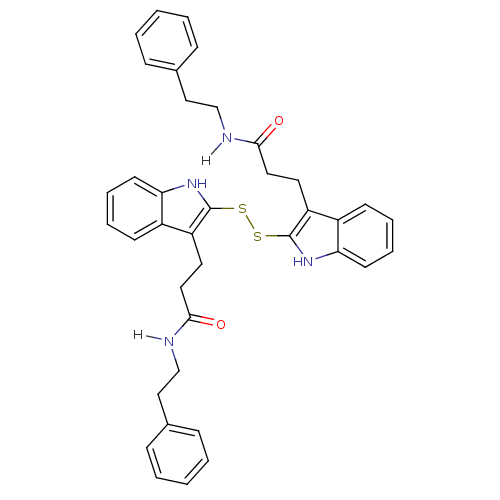 (1 H-indole-3-alkanamide deriv. 10o | N-(2-phenylet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4011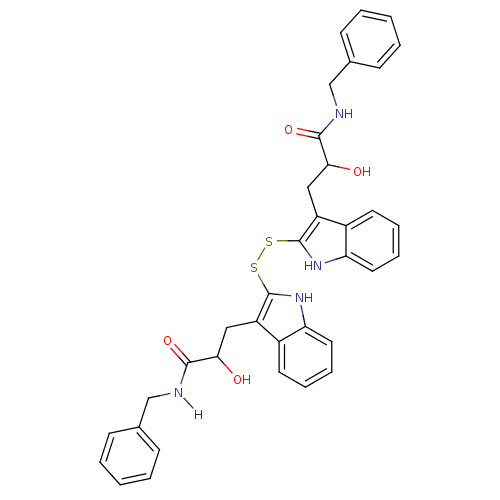 (1 H-indole-3-alkanamide deriv. 10t | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3996 (1 H-indole-3-alkanamide deriv. 10e | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3981 (1 H-indole-3-alkanamide deriv. 7 | Dimethyl 2,2 -D...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4014 (1 H-indole-3-alkanamide deriv. 11e | 2,3-Dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4006 (1 H-indole-3-alkanamide deriv. 10o | N-(2-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4000 (1 H-indole-3-alkanamide deriv. 10i | 2,2-Dithiobis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4010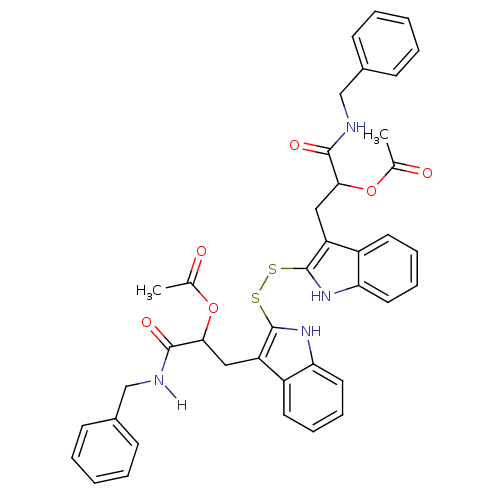 (1 H-indole-3-alkanamide deriv. 10s | 1-(benzylcarb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3991 (1 H-indole-3-alkanamide deriv. 10b | 2-(2-{[3-(cya...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3990 (1 H-indole-3-alkanamide deriv. 10a | N-benzyl-2-[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4002 (1 H-indole-3-alkanamide deriv. 10k | 2,2 -dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3994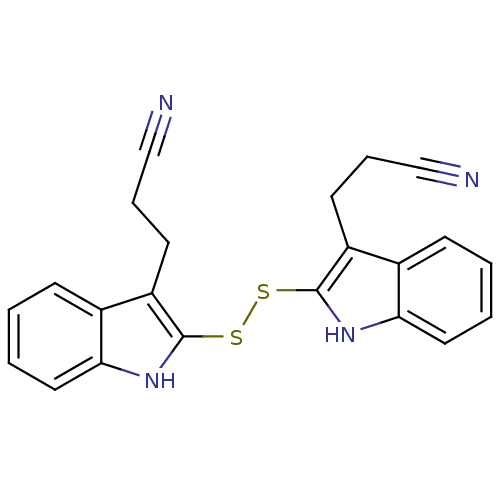 (1 H-indole-3-alkanamide deriv. 10c | 3-(2-{[3-(2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM3979 (2,2 -Dithiobis(1H-indole-3-propanoic acid) | 3-(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4007 (1 H-indole-3-alkanamide deriv. 10p | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3998 (1 H-indole-3-alkanamide deriv. 10g | N-methoxy-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3995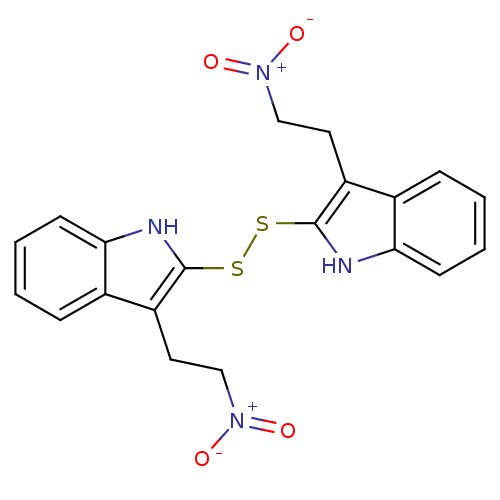 (1 H-indole-3-alkanamide deriv. 10d | 3-(2-nitroeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4008 (1 H-indole-3-alkanamide deriv. 10q | 2,2-Dithiobis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4004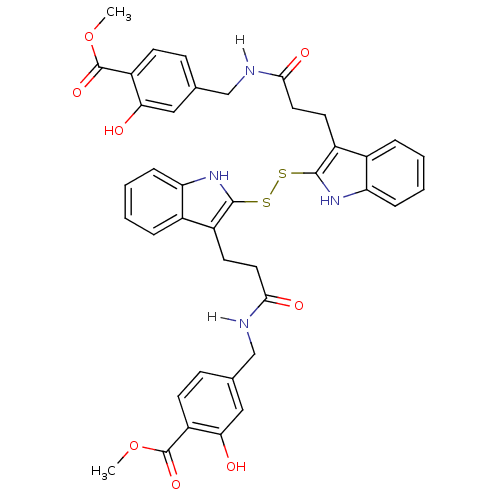 (1 H-indole-3-alkanamide deriv. 10m | 2,2 -Dithiobi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4013 (1 H-indole-3-alkanamide deriv. 11a | N-benzyl-2-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Src (Avian sarcoma virus) | BDBM4004 (1 H-indole-3-alkanamide deriv. 10m | 2,2 -Dithiobi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4015 (1 H-indole-3-alkanamide deriv. 11j | N-benzyl-3-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4012 (1 H-indole-3-alkanamide deriv. 10u | N-benzyl-4-[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 37: 598-609 (1994) Article DOI: 10.1021/jm00031a009 BindingDB Entry DOI: 10.7270/Q2MS3QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||