 Found 81 hits of Enzyme Inhibition Constant Data
Found 81 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50366775
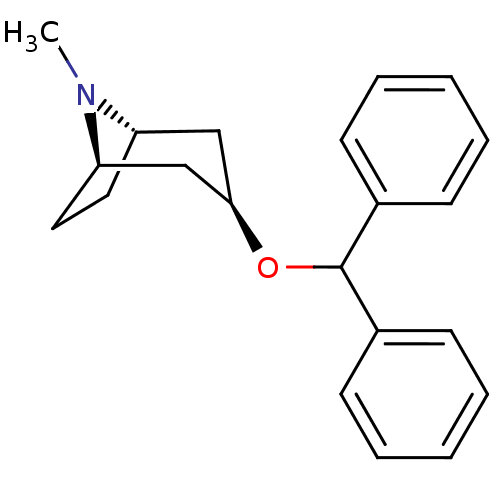
(BENZTROPINE | Benzatropine)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453903
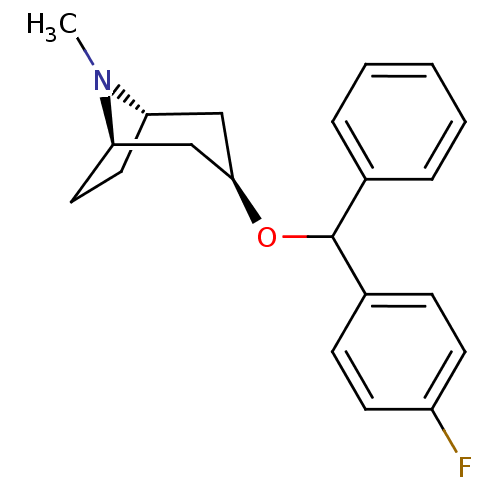
(CHEMBL3084883)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(F)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24FNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453899
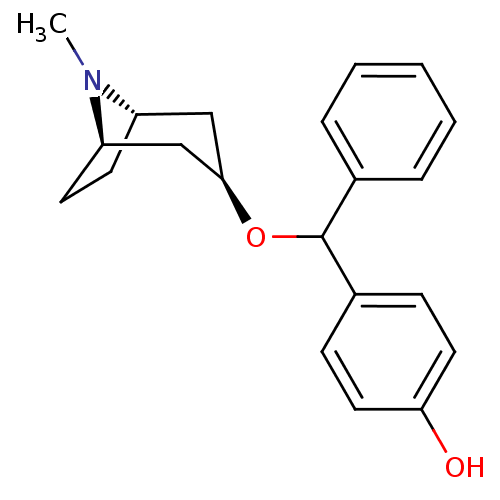
(CHEMBL3084872)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(O)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H25NO2/c1-22-17-9-10-18(22)14-20(13-17)24-21(15-5-3-2-4-6-15)16-7-11-19(23)12-8-16/h2-8,11-12,17-18,20-21,23H,9-10,13-14H2,1H3/t17-,18+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50366775

(BENZTROPINE | Benzatropine)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3/t18-,19+,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453908
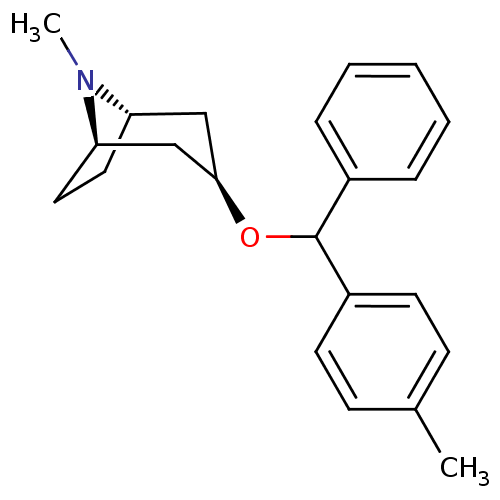
(CHEMBL3084881)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(C)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C22H27NO/c1-16-8-10-18(11-9-16)22(17-6-4-3-5-7-17)24-21-14-19-12-13-20(15-21)23(19)2/h3-11,19-22H,12-15H2,1-2H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453905
(CHEMBL3084882)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Br)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24BrNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453909
(CHEMBL3084880)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(OC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H27NO2/c1-23-18-10-11-19(23)15-21(14-18)25-22(16-6-4-3-5-7-16)17-8-12-20(24-2)13-9-17/h3-9,12-13,18-19,21-22H,10-11,14-15H2,1-2H3/t18-,19+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453906
(CHEMBL3084873)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C22H24F3NO/c1-26-18-11-12-19(26)14-20(13-18)27-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)22(23,24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86701
(3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccc(F)cc1)c1ccc(F)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H23F2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453907

(CHEMBL3084900)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C#N)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H24N2O/c1-24-19-11-12-20(24)14-21(13-19)25-22(17-5-3-2-4-6-17)18-9-7-16(15-23)8-10-18/h2-10,19-22H,11-14H2,1H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453911
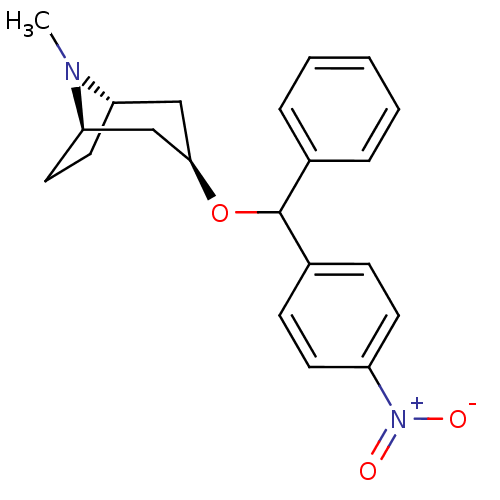
(CHEMBL3084899)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)[N+]([O-])=O)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H24N2O3/c1-22-18-11-12-19(22)14-20(13-18)26-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)23(24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM22165
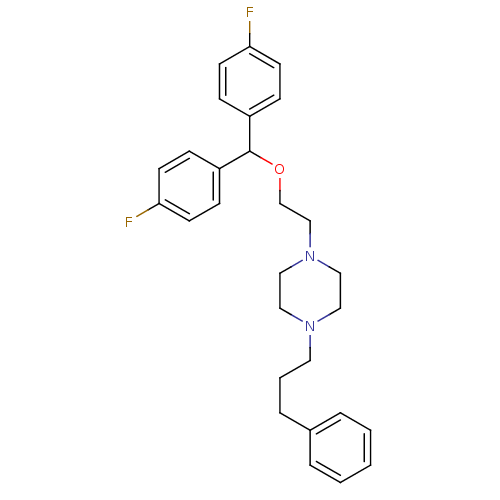
(1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...)Show SMILES Fc1ccc(cc1)C(OCCN1CCN(CCCc2ccccc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H32F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-3,5-6,8-15,28H,4,7,16-22H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM86701

(3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccc(F)cc1)c1ccc(F)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H23F2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453896

(CHEMBL3084898)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(CC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C23H29NO/c1-3-17-9-11-19(12-10-17)23(18-7-5-4-6-8-18)25-22-15-20-13-14-21(16-22)24(20)2/h4-12,20-23H,3,13-16H2,1-2H3/t20-,21+,22+,23? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453904
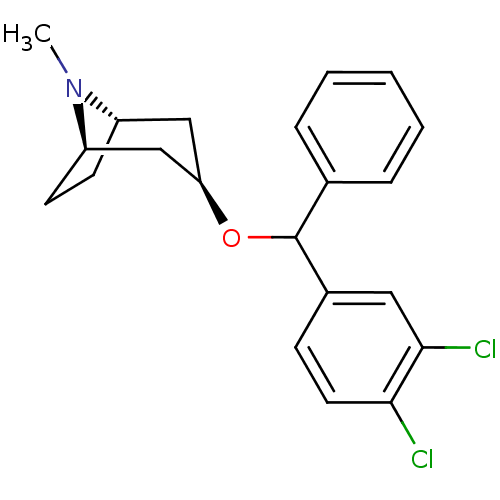
(CHEMBL3084892)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-16-8-9-17(24)13-18(12-16)25-21(14-5-3-2-4-6-14)15-7-10-19(22)20(23)11-15/h2-7,10-11,16-18,21H,8-9,12-13H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453903

(CHEMBL3084883)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(F)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24FNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453899

(CHEMBL3084872)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(O)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H25NO2/c1-22-17-9-10-18(22)14-20(13-17)24-21(15-5-3-2-4-6-15)16-7-11-19(23)12-8-16/h2-8,11-12,17-18,20-21,23H,9-10,13-14H2,1H3/t17-,18+,20+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453909

(CHEMBL3084880)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(OC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H27NO2/c1-23-18-10-11-19(23)15-21(14-18)25-22(16-6-4-3-5-7-16)17-8-12-20(24-2)13-9-17/h3-9,12-13,18-19,21-22H,10-11,14-15H2,1-2H3/t18-,19+,21+,22? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453910
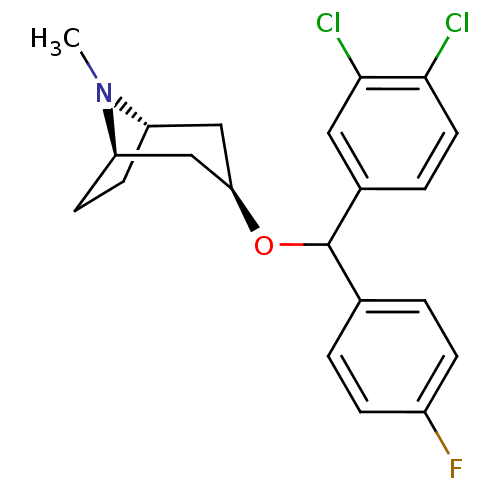
(CHEMBL3084896)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(F)cc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H22Cl2FNO/c1-25-16-7-8-17(25)12-18(11-16)26-21(13-2-5-15(24)6-3-13)14-4-9-19(22)20(23)10-14/h2-6,9-10,16-18,21H,7-8,11-12H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453902
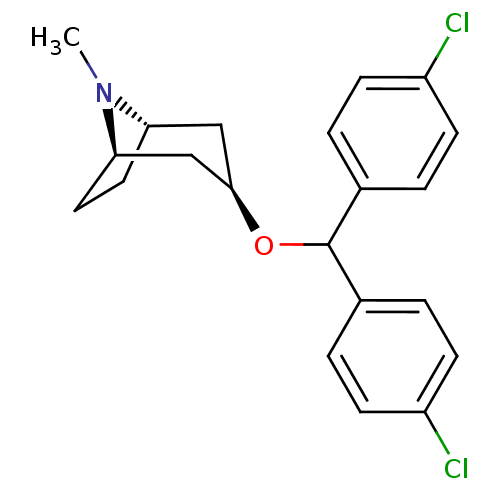
(CHEMBL3084884)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Cl)cc1)c1ccc(Cl)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453904

(CHEMBL3084892)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-16-8-9-17(24)13-18(12-16)25-21(14-5-3-2-4-6-14)15-7-10-19(22)20(23)11-15/h2-7,10-11,16-18,21H,8-9,12-13H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453903

(CHEMBL3084883)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(F)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24FNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50084717
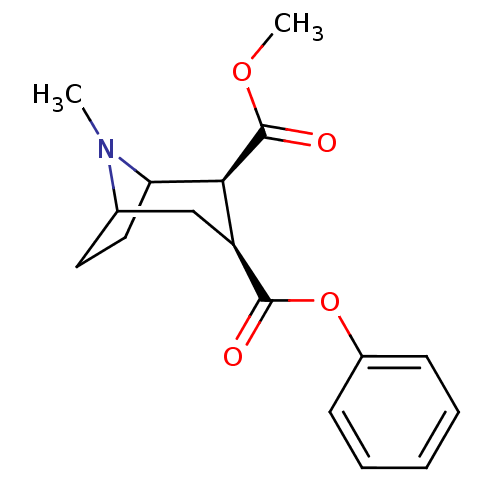
((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1C(=O)Oc1ccccc1)N2C |THB:2:4:20:6.7| Show InChI InChI=1S/C17H21NO4/c1-18-11-8-9-14(18)15(17(20)21-2)13(10-11)16(19)22-12-6-4-3-5-7-12/h3-7,11,13-15H,8-10H2,1-2H3/t11?,13-,14?,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453910

(CHEMBL3084896)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(F)cc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H22Cl2FNO/c1-25-16-7-8-17(25)12-18(11-16)26-21(13-2-5-15(24)6-3-13)14-4-9-19(22)20(23)10-14/h2-6,9-10,16-18,21H,7-8,11-12H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453902

(CHEMBL3084884)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Cl)cc1)c1ccc(Cl)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453905

(CHEMBL3084882)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Br)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24BrNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453908

(CHEMBL3084881)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(C)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C22H27NO/c1-16-8-10-18(11-9-16)22(17-6-4-3-5-7-17)24-21-14-19-12-13-20(15-21)23(19)2/h3-11,19-22H,12-15H2,1-2H3/t19-,20+,21+,22? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453905

(CHEMBL3084882)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Br)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24BrNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453901

(CHEMBL3084867)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(OC)cc1)c1ccc(OC)cc1)N2C |TLB:9:7:27:2.3| Show InChI InChI=1S/C23H29NO3/c1-24-18-8-9-19(24)15-22(14-18)27-23(16-4-10-20(25-2)11-5-16)17-6-12-21(26-3)13-7-17/h4-7,10-13,18-19,22-23H,8-9,14-15H2,1-3H3/t18-,19+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453911

(CHEMBL3084899)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)[N+]([O-])=O)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H24N2O3/c1-22-18-11-12-19(22)14-20(13-18)26-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)23(24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453898

(CHEMBL3084888)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(C)cc1)c1ccc(C)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C23H29NO/c1-16-4-8-18(9-5-16)23(19-10-6-17(2)7-11-19)25-22-14-20-12-13-21(15-22)24(20)3/h4-11,20-23H,12-15H2,1-3H3/t20-,21+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86701

(3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccc(F)cc1)c1ccc(F)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H23F2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453900

(CHEMBL3084866)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(C)(C)C)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C25H33NO/c1-25(2,3)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)27-23-16-21-14-15-22(17-23)26(21)4/h5-13,21-24H,14-17H2,1-4H3/t21-,22+,23+,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453897
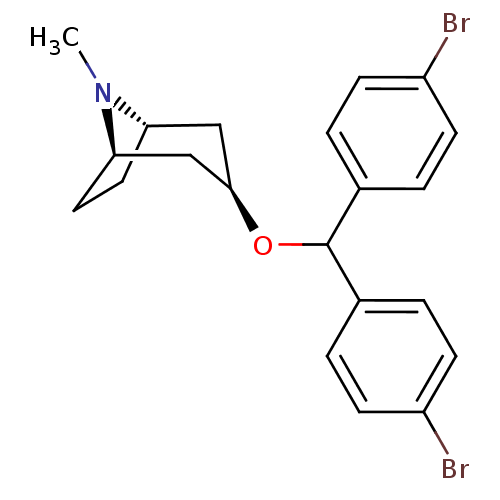
(CHEMBL3084897)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Br)cc1)c1ccc(Br)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Br2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453897

(CHEMBL3084897)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Br)cc1)c1ccc(Br)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Br2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453907

(CHEMBL3084900)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C#N)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H24N2O/c1-24-19-11-12-20(24)14-21(13-19)25-22(17-5-3-2-4-6-17)18-9-7-16(15-23)8-10-18/h2-10,19-22H,11-14H2,1H3/t19-,20+,21+,22? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453909

(CHEMBL3084880)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(OC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H27NO2/c1-23-18-10-11-19(23)15-21(14-18)25-22(16-6-4-3-5-7-16)17-8-12-20(24-2)13-9-17/h3-9,12-13,18-19,21-22H,10-11,14-15H2,1-2H3/t18-,19+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453897

(CHEMBL3084897)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Br)cc1)c1ccc(Br)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Br2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50366775

(BENZTROPINE | Benzatropine)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453904

(CHEMBL3084892)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-16-8-9-17(24)13-18(12-16)25-21(14-5-3-2-4-6-14)15-7-10-19(22)20(23)11-15/h2-7,10-11,16-18,21H,8-9,12-13H2,1H3/t16-,17+,18+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50453900

(CHEMBL3084866)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(C)(C)C)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C25H33NO/c1-25(2,3)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)27-23-16-21-14-15-22(17-23)26(21)4/h5-13,21-24H,14-17H2,1-4H3/t21-,22+,23+,24? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453908

(CHEMBL3084881)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(C)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C22H27NO/c1-16-8-10-18(11-9-16)22(17-6-4-3-5-7-17)24-21-14-19-12-13-20(15-21)23(19)2/h3-11,19-22H,12-15H2,1-2H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453907

(CHEMBL3084900)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C#N)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H24N2O/c1-24-19-11-12-20(24)14-21(13-19)25-22(17-5-3-2-4-6-17)18-9-7-16(15-23)8-10-18/h2-10,19-22H,11-14H2,1H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453911

(CHEMBL3084899)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)[N+]([O-])=O)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H24N2O3/c1-22-18-11-12-19(22)14-20(13-18)26-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)23(24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453899

(CHEMBL3084872)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(O)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H25NO2/c1-22-17-9-10-18(22)14-20(13-17)24-21(15-5-3-2-4-6-15)16-7-11-19(23)12-8-16/h2-8,11-12,17-18,20-21,23H,9-10,13-14H2,1H3/t17-,18+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453910

(CHEMBL3084896)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(F)cc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H22Cl2FNO/c1-25-16-7-8-17(25)12-18(11-16)26-21(13-2-5-15(24)6-3-13)14-4-9-19(22)20(23)10-14/h2-6,9-10,16-18,21H,7-8,11-12H2,1H3/t16-,17+,18+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453898

(CHEMBL3084888)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(C)cc1)c1ccc(C)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C23H29NO/c1-16-4-8-18(9-5-16)23(19-10-6-17(2)7-11-19)25-22-14-20-12-13-21(15-22)24(20)3/h4-11,20-23H,12-15H2,1-3H3/t20-,21+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453896

(CHEMBL3084898)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(CC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C23H29NO/c1-3-17-9-11-19(12-10-17)23(18-7-5-4-6-8-18)25-22-15-20-13-14-21(16-22)24(20)2/h4-12,20-23H,3,13-16H2,1-2H3/t20-,21+,22+,23? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453906

(CHEMBL3084873)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C22H24F3NO/c1-26-18-11-12-19(26)14-20(13-18)27-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)22(23,24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 635 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453896

(CHEMBL3084898)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(CC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C23H29NO/c1-3-17-9-11-19(12-10-17)23(18-7-5-4-6-8-18)25-22-15-20-13-14-21(16-22)24(20)2/h4-12,20-23H,3,13-16H2,1-2H3/t20-,21+,22+,23? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 703 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453898

(CHEMBL3084888)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(C)cc1)c1ccc(C)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C23H29NO/c1-16-4-8-18(9-5-16)23(19-10-6-17(2)7-11-19)25-22-14-20-12-13-21(15-22)24(20)3/h4-11,20-23H,12-15H2,1-3H3/t20-,21+,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 716 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453901

(CHEMBL3084867)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(OC)cc1)c1ccc(OC)cc1)N2C |TLB:9:7:27:2.3| Show InChI InChI=1S/C23H29NO3/c1-24-18-8-9-19(24)15-22(14-18)27-23(16-4-10-20(25-2)11-5-16)17-6-12-21(26-3)13-7-17/h4-7,10-13,18-19,22-23H,8-9,14-15H2,1-3H3/t18-,19+,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453902

(CHEMBL3084884)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Cl)cc1)c1ccc(Cl)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50453906

(CHEMBL3084873)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C22H24F3NO/c1-26-18-11-12-19(26)14-20(13-18)27-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)22(23,24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453900

(CHEMBL3084866)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(C)(C)C)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C25H33NO/c1-25(2,3)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)27-23-16-21-14-15-22(17-23)26(21)4/h5-13,21-24H,14-17H2,1-4H3/t21-,22+,23+,24? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453901

(CHEMBL3084867)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(OC)cc1)c1ccc(OC)cc1)N2C |TLB:9:7:27:2.3| Show InChI InChI=1S/C23H29NO3/c1-24-18-8-9-19(24)15-22(14-18)27-23(16-4-10-20(25-2)11-5-16)17-6-12-21(26-3)13-7-17/h4-7,10-13,18-19,22-23H,8-9,14-15H2,1-3H3/t18-,19+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453910

(CHEMBL3084896)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(F)cc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H22Cl2FNO/c1-25-16-7-8-17(25)12-18(11-16)26-21(13-2-5-15(24)6-3-13)14-4-9-19(22)20(23)10-14/h2-6,9-10,16-18,21H,7-8,11-12H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453905

(CHEMBL3084882)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Br)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24BrNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453897

(CHEMBL3084897)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Br)cc1)c1ccc(Br)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Br2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453904

(CHEMBL3084892)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-16-8-9-17(24)13-18(12-16)25-21(14-5-3-2-4-6-14)15-7-10-19(22)20(23)11-15/h2-7,10-11,16-18,21H,8-9,12-13H2,1H3/t16-,17+,18+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453903

(CHEMBL3084883)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(F)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H24FNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM86701

(3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccc(F)cc1)c1ccc(F)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H23F2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453902

(CHEMBL3084884)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Cl)cc1)c1ccc(Cl)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C21H23Cl2NO/c1-24-18-10-11-19(24)13-20(12-18)25-21(14-2-6-16(22)7-3-14)15-4-8-17(23)9-5-15/h2-9,18-21H,10-13H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453911

(CHEMBL3084899)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)[N+]([O-])=O)N2C |TLB:9:7:26:3.2| Show InChI InChI=1S/C21H24N2O3/c1-22-18-11-12-19(22)14-20(13-18)26-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)23(24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453907

(CHEMBL3084900)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C#N)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H24N2O/c1-24-19-11-12-20(24)14-21(13-19)25-22(17-5-3-2-4-6-17)18-9-7-16(15-23)8-10-18/h2-10,19-22H,11-14H2,1H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50366775

(BENZTROPINE | Benzatropine)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3/t18-,19+,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453909

(CHEMBL3084880)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(OC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C22H27NO2/c1-23-18-10-11-19(23)15-21(14-18)25-22(16-6-4-3-5-7-16)17-8-12-20(24-2)13-9-17/h3-9,12-13,18-19,21-22H,10-11,14-15H2,1-2H3/t18-,19+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453908

(CHEMBL3084881)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(C)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C22H27NO/c1-16-8-10-18(11-9-16)22(17-6-4-3-5-7-17)24-21-14-19-12-13-20(15-21)23(19)2/h3-11,19-22H,12-15H2,1-2H3/t19-,20+,21+,22? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453899

(CHEMBL3084872)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(O)cc1)N2C |TLB:9:7:24:3.2| Show InChI InChI=1S/C21H25NO2/c1-22-17-9-10-18(22)14-20(13-17)24-21(15-5-3-2-4-6-15)16-7-11-19(23)12-8-16/h2-8,11-12,17-18,20-21,23H,9-10,13-14H2,1H3/t17-,18+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 677 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453896

(CHEMBL3084898)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(CC)cc1)N2C |TLB:9:7:25:3.2| Show InChI InChI=1S/C23H29NO/c1-3-17-9-11-19(12-10-17)23(18-7-5-4-6-8-18)25-22-15-20-13-14-21(16-22)24(20)2/h4-12,20-23H,3,13-16H2,1-2H3/t20-,21+,22+,23? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 984 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453906

(CHEMBL3084873)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C22H24F3NO/c1-26-18-11-12-19(26)14-20(13-18)27-21(15-5-3-2-4-6-15)16-7-9-17(10-8-16)22(23,24)25/h2-10,18-21H,11-14H2,1H3/t18-,19+,20+,21? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453898

(CHEMBL3084888)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(C)cc1)c1ccc(C)cc1)N2C |TLB:9:7:25:2.3| Show InChI InChI=1S/C23H29NO/c1-16-4-8-18(9-5-16)23(19-10-6-17(2)7-11-19)25-22-14-20-12-13-21(15-22)24(20)3/h4-11,20-23H,12-15H2,1-3H3/t20-,21+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453901

(CHEMBL3084867)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(OC)cc1)c1ccc(OC)cc1)N2C |TLB:9:7:27:2.3| Show InChI InChI=1S/C23H29NO3/c1-24-18-8-9-19(24)15-22(14-18)27-23(16-4-10-20(25-2)11-5-16)17-6-12-21(26-3)13-7-17/h4-7,10-13,18-19,22-23H,8-9,14-15H2,1-3H3/t18-,19+,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM86702

(3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccc(Cl)cc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H24ClNO/c1-23-18-11-12-19(23)14-20(13-18)24-21(15-5-3-2-4-6-15)16-7-9-17(22)10-8-16/h2-10,18-21H,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453900

(CHEMBL3084866)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccccc1)c1ccc(cc1)C(C)(C)C)N2C |TLB:9:7:27:3.2| Show InChI InChI=1S/C25H33NO/c1-25(2,3)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)27-23-16-21-14-15-22(17-23)26(21)4/h5-13,21-24H,14-17H2,1-4H3/t21-,22+,23+,24? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake in rat caudate putamen. |
J Med Chem 38: 3933-40 (1995)
BindingDB Entry DOI: 10.7270/Q20V8DF2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data