

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230748 (CHEMBL298087) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230728 (CHEMBL297274) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50127140 ((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230743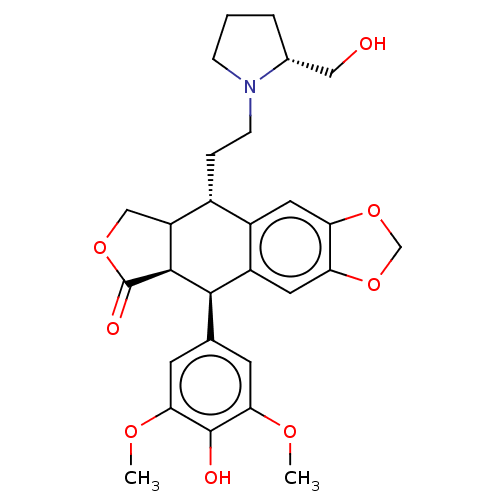 (CHEMBL541042) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230731 (CHEMBL1204204) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230746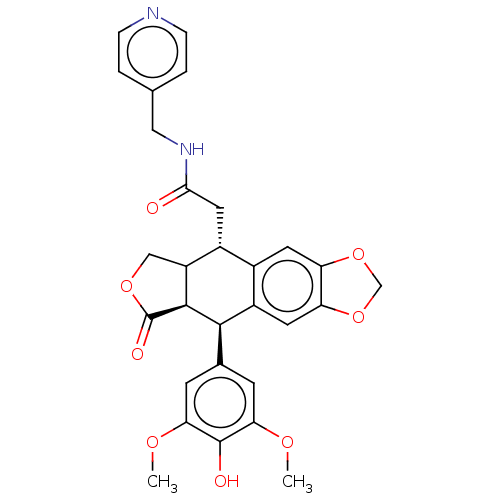 (CHEMBL554198) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230730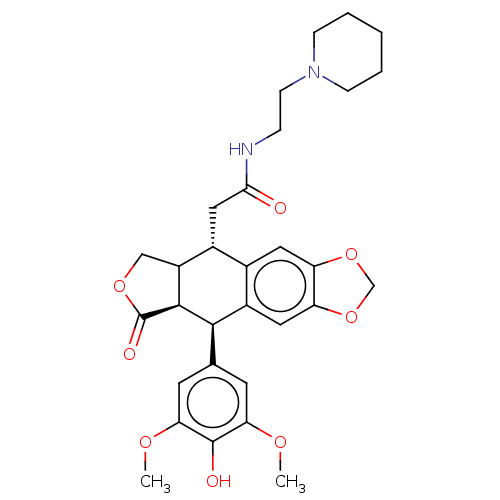 (CHEMBL553647) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230729 (CHEMBL1202604) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230742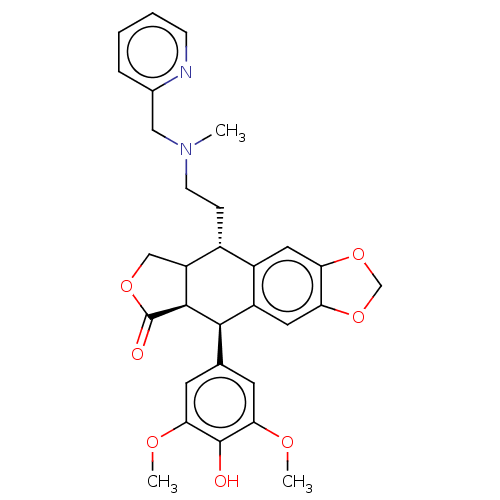 (CHEMBL1202600) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230740 (CHEMBL1202605) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230738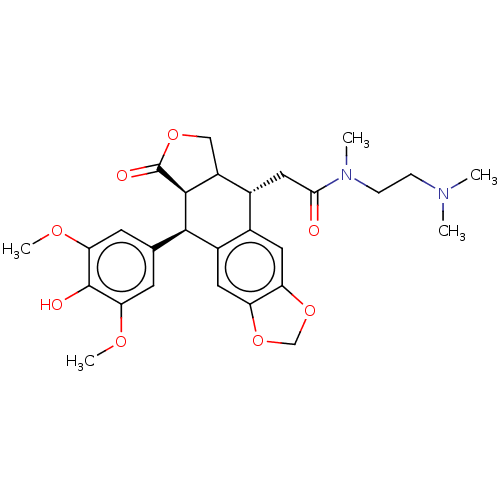 (CHEMBL544018) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230754 (CHEMBL538490) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230735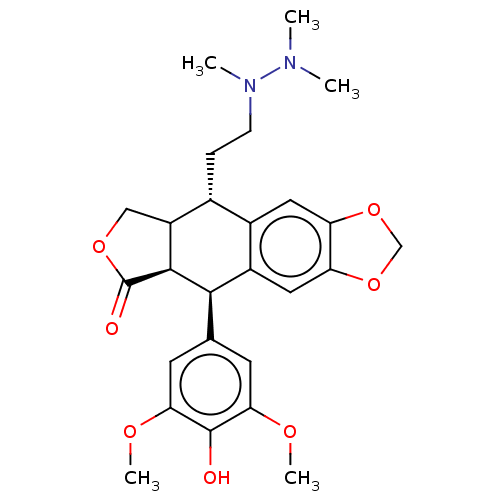 (CHEMBL541545) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230756 (CHEMBL1202598) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230750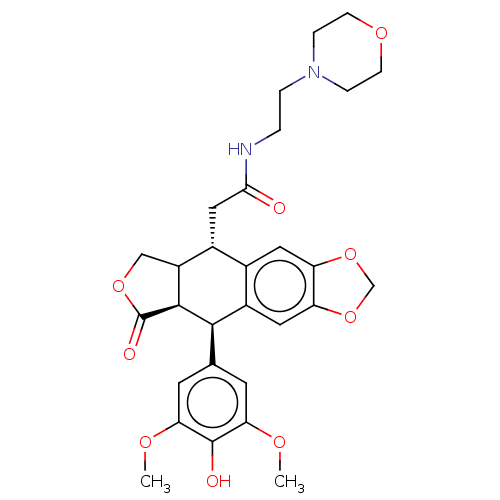 (CHEMBL540528) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230737 (CHEMBL1202595) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230732 (CHEMBL541807) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230751 (CHEMBL556408) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230725 (CHEMBL538240) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230752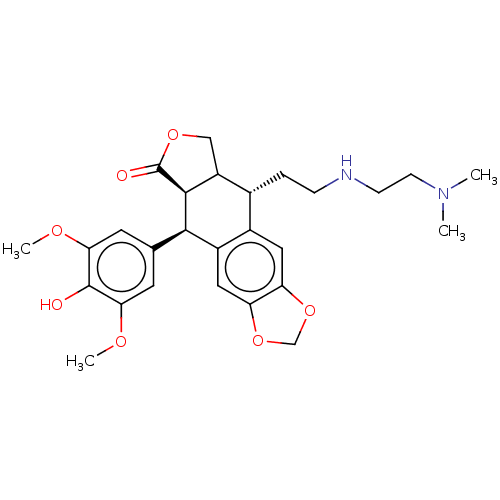 (CHEMBL1202603) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230753 (CHEMBL553550) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230755 (CHEMBL539255) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230724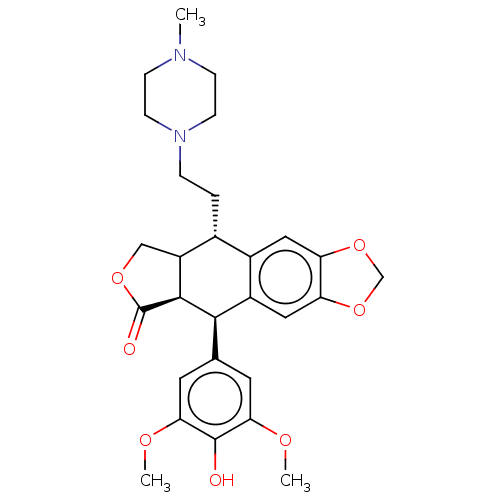 (CHEMBL1202602) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230736 (CHEMBL1202599) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230749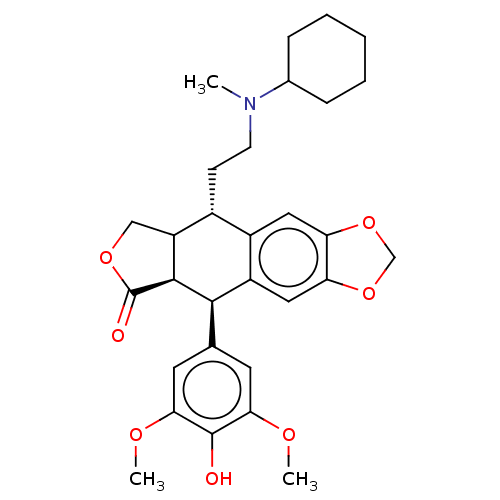 (CHEMBL539511) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230739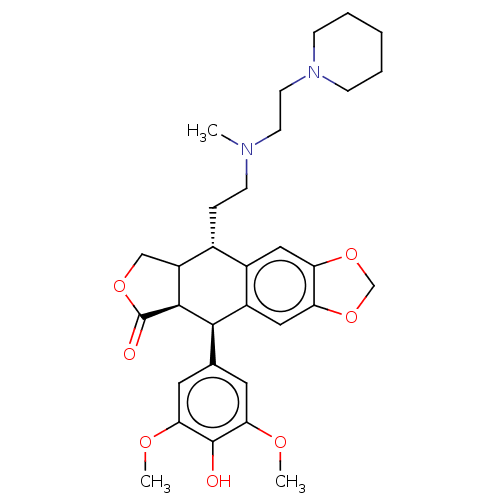 (CHEMBL1202597) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230747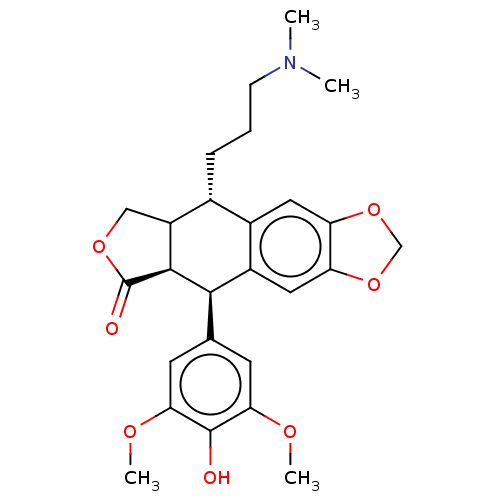 (CHEMBL557621) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230741 (CHEMBL556053) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230745 (CHEMBL1202601) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230727 (CHEMBL552576) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230726 (CHEMBL555319) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230734 (CHEMBL540275) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230744 (CHEMBL543789) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50230733 (CHEMBL1202596) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Drug concentration needed to produce a 50% reduction of bovine brain tubulin polymerization relative to the control | J Med Chem 36: 1689-99 (1993) BindingDB Entry DOI: 10.7270/Q2TB194D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||