

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM345819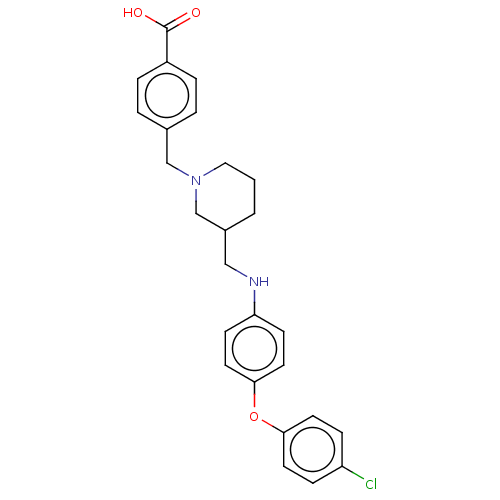 (US10202362, Compound T2.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc. US Patent | Assay Description LTB4 formed was quantified with the HTRF assay in which free LTB4 competes with LTB4-XL665 conjugate (acceptor) for anti-LTB4 monoclonal antibody lab... | US Patent US10202362 (2019) BindingDB Entry DOI: 10.7270/Q2MC9241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM345818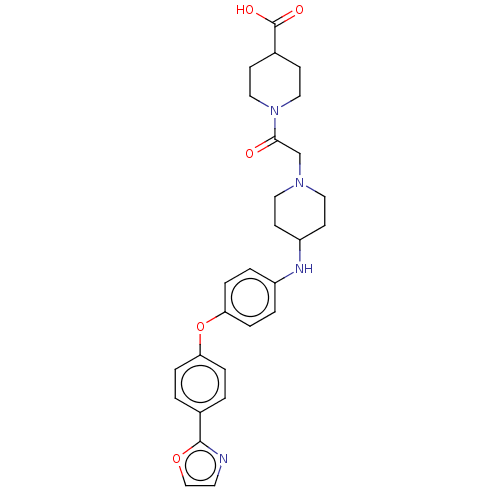 (US10202362, Compound T2.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc. US Patent | Assay Description LTB4 formed was quantified with the HTRF assay in which free LTB4 competes with LTB4-XL665 conjugate (acceptor) for anti-LTB4 monoclonal antibody lab... | US Patent US10202362 (2019) BindingDB Entry DOI: 10.7270/Q2MC9241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM345818 (US10202362, Compound T2.1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc. US Patent | Assay Description In brief, the enzyme (29 nM) was incubated with L-alanine-p-nitroanilide (1 mM) in 50 mM HEPES (pH 7.5), 100 mM KCL, 1% DMSO in the absence or presen... | US Patent US10202362 (2019) BindingDB Entry DOI: 10.7270/Q2MC9241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM345819 (US10202362, Compound T2.2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc. US Patent | Assay Description In brief, the enzyme (29 nM) was incubated with L-alanine-p-nitroanilide (1 mM) in 50 mM HEPES (pH 7.5), 100 mM KCL, 1% DMSO in the absence or presen... | US Patent US10202362 (2019) BindingDB Entry DOI: 10.7270/Q2MC9241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||