

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242915 (US9428447, DK-406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 557 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242910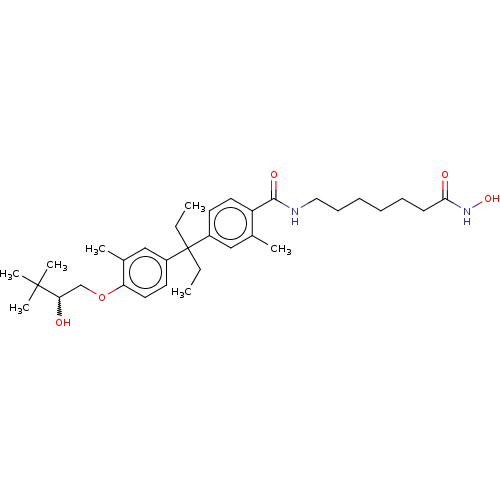 (US9428447, DK-362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242912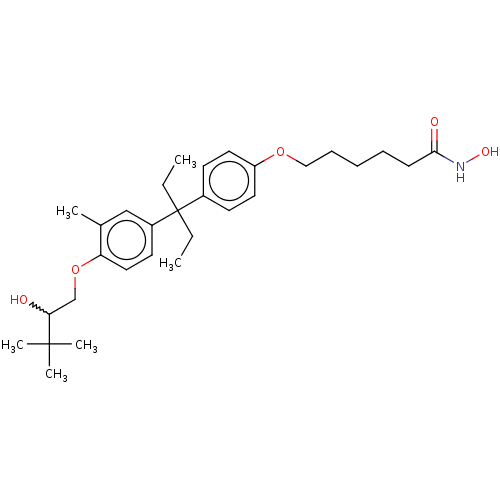 (US9428447, DK-367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242914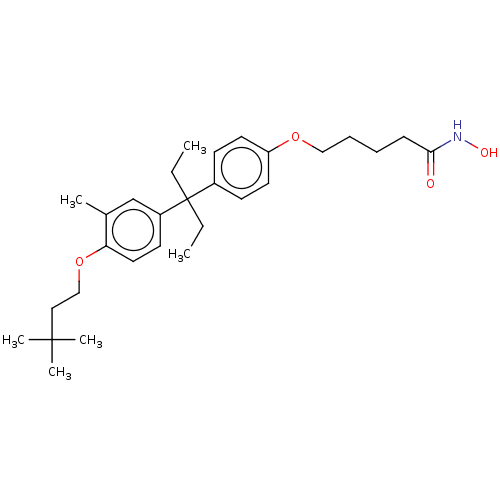 (US9428447, DK-405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242906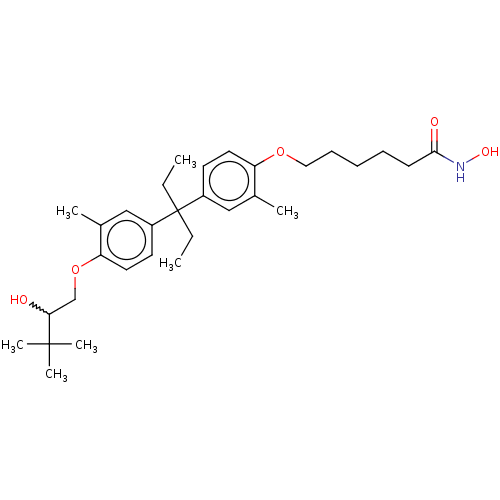 (US9428447, DK-319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242913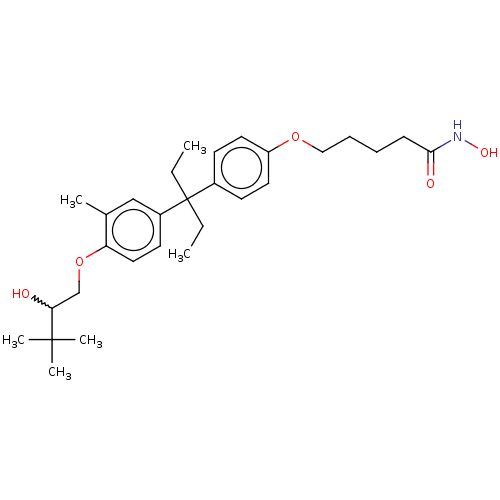 (US9428447, DK-381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242909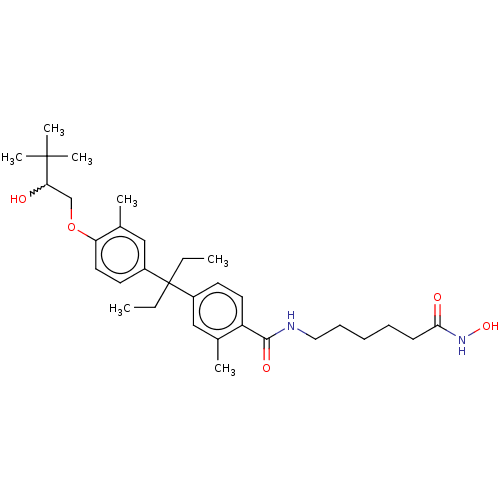 (US9428447, DK-361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242908 (US9428447, DK-347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242916 (US9428447, JF-B06) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242907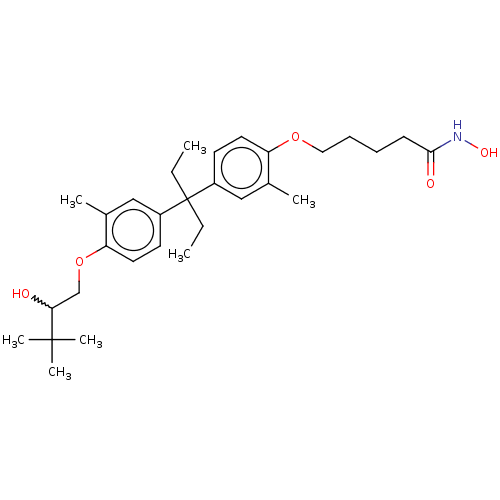 (US9428447, DK-320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242911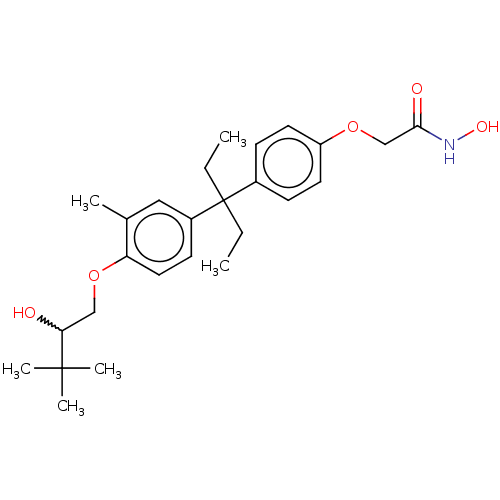 (US9428447, DK-366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242901 (US9428447, JFD-15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.83E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242899 (US9428447, JF-B53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242898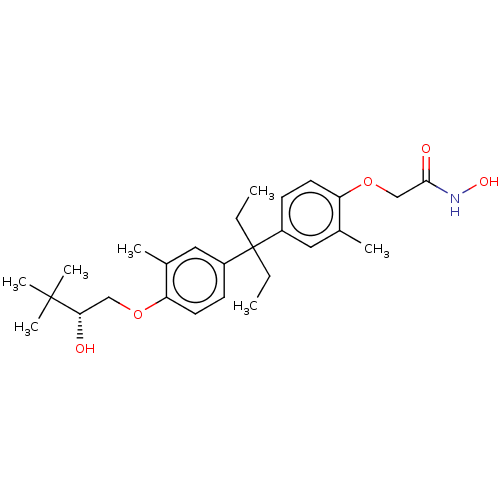 (US9428447, JF-C71) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242903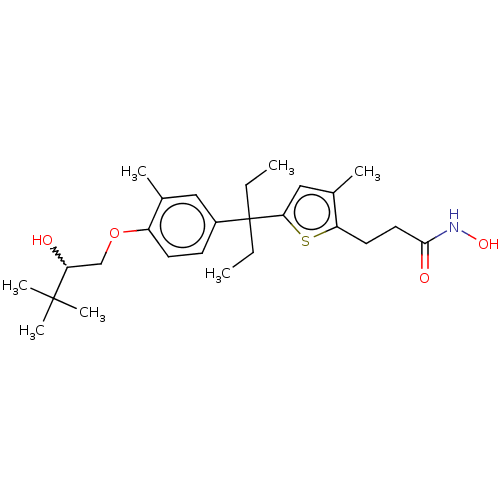 (US9428447, DK-178) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242898 (US9428447, JF-C71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242905 (US9428447, DK-305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242900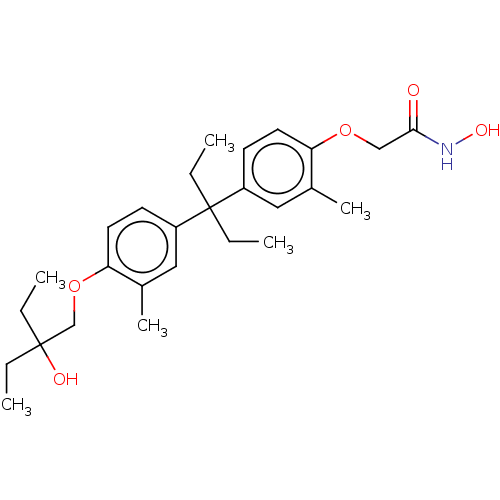 (US9428447, JF-B54) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242904 (US9428447, DK-201) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242900 (US9428447, JF-B54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242899 (US9428447, JF-B53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242917 (US9428447, JF-B21) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242902 (US9428447, JFD-50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242917 (US9428447, JF-B21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242902 (US9428447, JFD-50) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242901 (US9428447, JFD-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.71E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242903 (US9428447, DK-178) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.73E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||