

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314731 (US9611270, Example 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314735 (US9611270, Example 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50358201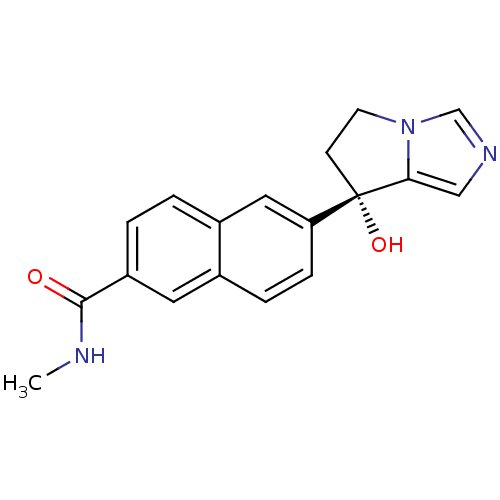 (CHEMBL1921976 | US9611270, orteronel) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50358201 (CHEMBL1921976 | US9611270, orteronel) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314733 (US9611270, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314740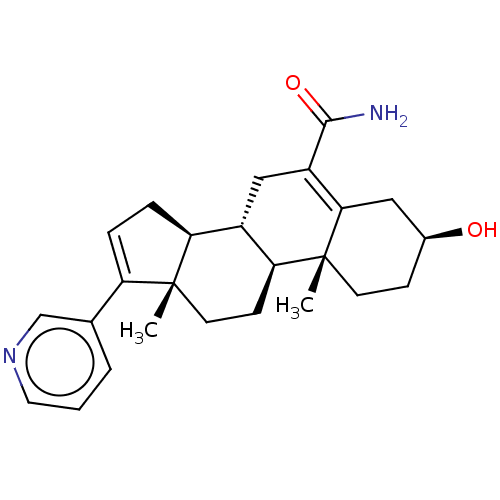 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314736 (US9611270, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314740 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314736 (US9611270, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314739 (US9611270, Example 39 | US9611270, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314734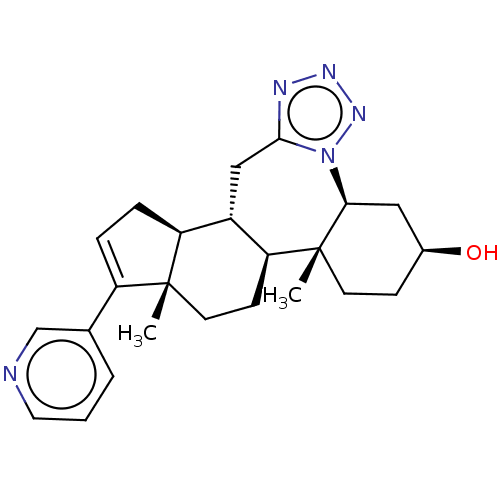 (US9611270, Example 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314732 (US9611270, Example 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314737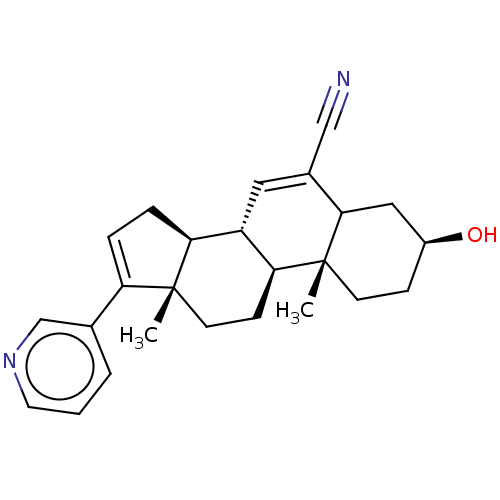 (US9611270, Example 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50435990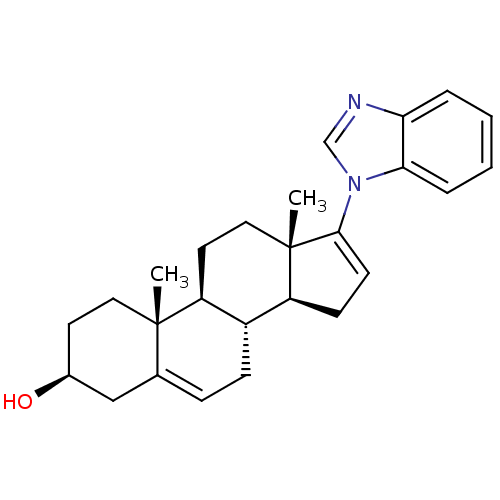 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50435990 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM314730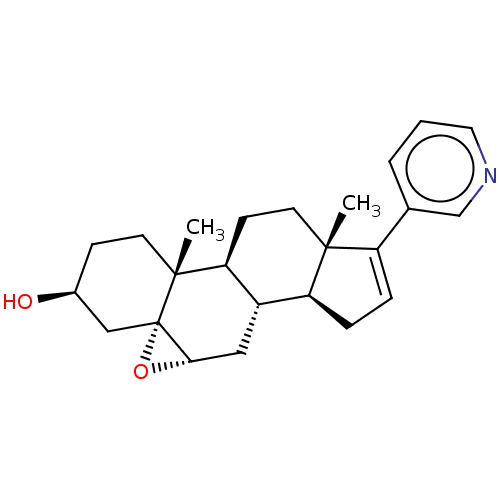 (US9611270, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314739 (US9611270, Example 39 | US9611270, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 831 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||