

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125050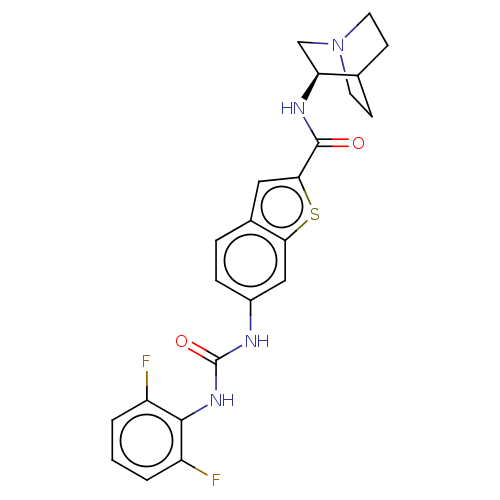 (US8772511, 11 | US9714242, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125047 (US8772511, 4 | US9714242, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125047 (US8772511, 4 | US9714242, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125051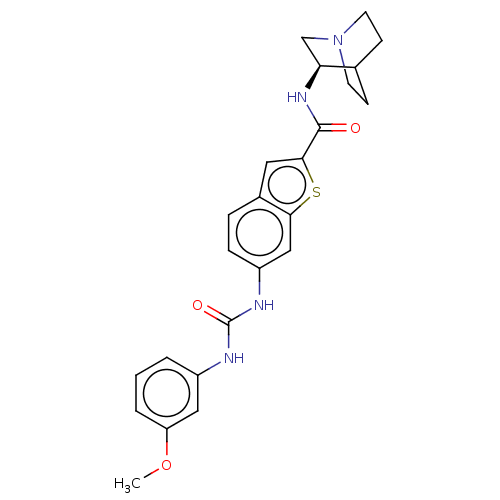 (US8772511, 16 | US9714242, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125049 (US8772511, 6 | US9714242, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125052 (US8772511, 21 | US9714242, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM125048 (US8772511, 5 | US9714242, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol, 1999, 38, 679-690.Rat brain t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM125052 (US8772511, 21 | US9714242, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM125049 (US8772511, 6 | US9714242, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM125051 (US8772511, 16 | US9714242, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM125050 (US8772511, 11 | US9714242, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM264253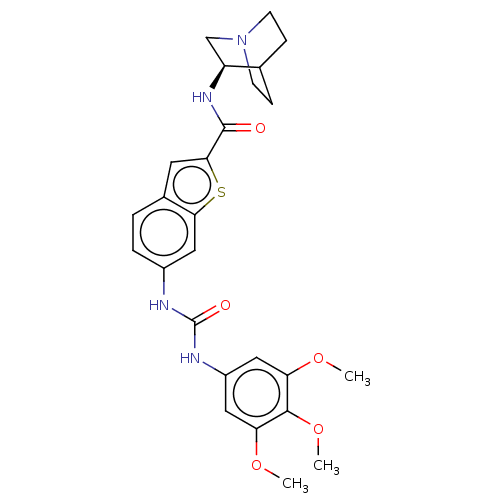 (N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-6-({[(3,4,5-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM125048 (US8772511, 5 | US9714242, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Membranes from HEK293 cells which express recombinant human 5-HT3 receptor (RB-HS3, Receptor Biology, Inc., MD, USA) are diluted in accordance with t... | US Patent US9714242 (2017) BindingDB Entry DOI: 10.7270/Q2M61N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||