

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Monoglyceride lipase | ||
| Ligand | BDBM581632 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Biological Assay | ||
| IC50 | 1.50±n/a nM | ||
| Citation |  Alcazar, J; Ameriks, MK; Berry, CB; Garcia-Reynaga, P; Samant, AV; Vega-Ramiro, JA Azaspirocycles as monoacylglycerol lipase modulators US Patent US11505546 Publication Date 11/22/2022 Alcazar, J; Ameriks, MK; Berry, CB; Garcia-Reynaga, P; Samant, AV; Vega-Ramiro, JA Azaspirocycles as monoacylglycerol lipase modulators US Patent US11505546 Publication Date 11/22/2022 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Monoglyceride lipase | |||
| Name: | Monoglyceride lipase | ||
| Synonyms: | HU-K5 | Lysophospholipase homolog | Lysophospholipase-like | MAGL | MGL | MGLL | MGLL_HUMAN | ||
| Type: | Hydrolase | ||
| Mol. Mass.: | 33264.56 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Human recombinant MGL (Cayman Chemical, cat# 10008354). | ||
| Residue: | 303 | ||
| Sequence: |
| ||
| BDBM581632 | |||
| n/a | |||
| Name | BDBM581632 | ||
| Synonyms: | (2r,4S*)-2-((R*)-6-(4-(Trifluoromethyl)phenyl)-2-azaspiro[3.4]octane-2- carbonyl)-5-azaspiro[3.4]octan-6-one | US11505546, Example 47 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C21H23F3N2O3 | ||
| Mol. Mass. | 408.4141 | ||
| SMILES | FC(F)(F)c1ccc(cc1)[C@@H]1CCC2(CN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)C1 |r,wU:19.20,wD:10.10,21.23,(9.2,.19,;8.55,-1.21,;9.43,-2.47,;10.08,-1.07,;7.01,-1.35,;6.36,-2.74,;4.83,-2.88,;3.94,-1.62,;4.59,-.22,;6.13,-.09,;2.4,-1.62,;1.5,-2.86,;.03,-2.39,;.03,-.85,;-.21,.67,;-1.73,.43,;-1.49,-1.09,;-2.97,1.34,;-2.97,2.88,;-4.38,.71,;-4.78,-.78,;-6.27,-.38,;-5.87,1.11,;-6.19,-1.92,;-7.62,-2.47,;-8.59,-1.27,;-10.08,-1.07,;-7.75,.02,;1.5,-.37,)| | ||
| Structure | 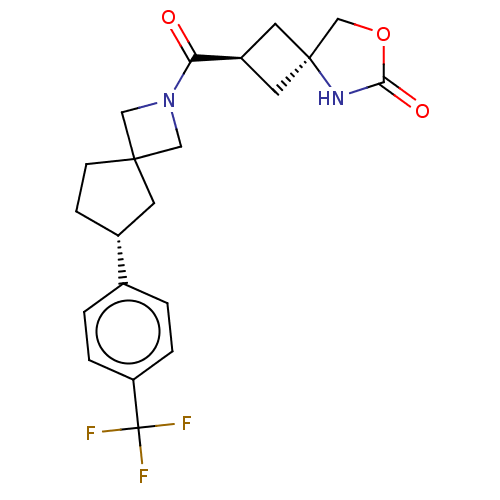
| ||