| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin D |
|---|
| Ligand | BDBM171454 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In Vitro Enzymatic FRET Assay |
|---|
| IC50 | 301500±n/a nM |
|---|
| Citation |  Minatti, AE; Low, JD; Allen, JR; Amegadzie, A; Brown, J; Frohn, MJ; Guzman-Perez, A; Harrington, PE; Lopez, P; Ma, VV; Nishimura, N; Qian, W; Rumfelt, S; Rzasa, RM; Sham, K; Smith, AL; White, R; Xue, Q Perfluorinated cyclopropyl fused 1,3-oxazin-2-amine compounds as beta-secretase inhibitors and methods of use US Patent US9611261 Publication Date 4/4/2017 Minatti, AE; Low, JD; Allen, JR; Amegadzie, A; Brown, J; Frohn, MJ; Guzman-Perez, A; Harrington, PE; Lopez, P; Ma, VV; Nishimura, N; Qian, W; Rumfelt, S; Rzasa, RM; Sham, K; Smith, AL; White, R; Xue, Q Perfluorinated cyclopropyl fused 1,3-oxazin-2-amine compounds as beta-secretase inhibitors and methods of use US Patent US9611261 Publication Date 4/4/2017 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin D |
|---|
| Name: | Cathepsin D |
|---|
| Synonyms: | CATD_HUMAN | CPSD | CTSD | Cathepsin D [Precursor] | Cathepsin D heavy chain | Cathepsin D light chain | Cathepsin D precursor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 44551.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human proCathepsin D (SwissProt accession number P07339) was expressed in Sf9 cells, purified, and autoactivated. |
|---|
| Residue: | 412 |
|---|
| Sequence: | MQPSSLLPLALCLLAAPASALVRIPLHKFTSIRRTMSEVGGSVEDLIAKGPVSKYSQAVP
AVTEGPIPEVLKNYMDAQYYGEIGIGTPPQCFTVVFDTGSSNLWVPSIHCKLLDIACWIH
HKYNSDKSSTYVKNGTSFDIHYGSGSLSGYLSQDTVSVPCQSASSASALGGVKVERQVFG
EATKQPGITFIAAKFDGILGMAYPRISVNNVLPVFDNLMQQKLVDQNIFSFYLSRDPDAQ
PGGELMLGGTDSKYYKGSLSYLNVTRKAYWQVHLDQVEVASGLTLCKEGCEAIVDTGTSL
MVGPVDEVRELQKAIGAVPLIQGEYMIPCEKVSTLPAITLKLGGKGYKLSPEDYTLKVSQ
AGKTLCLSGFMGMDIPPPSGPLWILGDVFIGRYYTVFDRDNNRVGFAEAARL
|
|
|
|---|
| BDBM171454 |
|---|
| n/a |
|---|
| Name | BDBM171454 |
|---|
| Synonyms: | US9085576, 88 | US9611261, Example 88 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H14ClF2N5O2 |
|---|
| Mol. Mass. | 417.797 |
|---|
| SMILES | NC1=N[C@@](CF)([C@H]2C[C@H]2O1)c1cc(NC(=O)c2ccc(cn2)C#N)cc(Cl)c1F |r,t:1| |
|---|
| Structure | 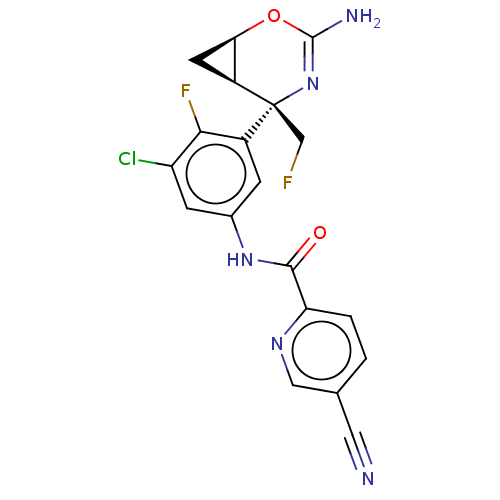
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Minatti, AE; Low, JD; Allen, JR; Amegadzie, A; Brown, J; Frohn, MJ; Guzman-Perez, A; Harrington, PE; Lopez, P; Ma, VV; Nishimura, N; Qian, W; Rumfelt, S; Rzasa, RM; Sham, K; Smith, AL; White, R; Xue, Q Perfluorinated cyclopropyl fused 1,3-oxazin-2-amine compounds as beta-secretase inhibitors and methods of use US Patent US9611261 Publication Date 4/4/2017
Minatti, AE; Low, JD; Allen, JR; Amegadzie, A; Brown, J; Frohn, MJ; Guzman-Perez, A; Harrington, PE; Lopez, P; Ma, VV; Nishimura, N; Qian, W; Rumfelt, S; Rzasa, RM; Sham, K; Smith, AL; White, R; Xue, Q Perfluorinated cyclopropyl fused 1,3-oxazin-2-amine compounds as beta-secretase inhibitors and methods of use US Patent US9611261 Publication Date 4/4/2017