

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Glutamate receptor ionotropic, NMDA 2B | ||
| Ligand | BDBM50269754 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_1707178 | ||
| IC50 | 16±n/a nM | ||
| Citation |  Anan, K; Masui, M; Hara, S; Ohara, M; Kume, M; Yamamoto, S; Shinohara, S; Tsuji, H; Shimada, S; Yagi, S; Hasebe, N; Kai, H Discovery of orally bioavailable cyclohexanol-based NR2B-selective NMDA receptor antagonists with analgesic activity utilizing a scaffold hopping approach. Bioorg Med Chem Lett27:4194-4198 (2017) [PubMed] Article Anan, K; Masui, M; Hara, S; Ohara, M; Kume, M; Yamamoto, S; Shinohara, S; Tsuji, H; Shimada, S; Yagi, S; Hasebe, N; Kai, H Discovery of orally bioavailable cyclohexanol-based NR2B-selective NMDA receptor antagonists with analgesic activity utilizing a scaffold hopping approach. Bioorg Med Chem Lett27:4194-4198 (2017) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Glutamate receptor ionotropic, NMDA 2B | |||
| Name: | Glutamate receptor ionotropic, NMDA 2B | ||
| Synonyms: | GluN2B | Glutamate [NMDA] receptor subunit epsilon-2 | Glutamate receptor ionotropic, NMDA 2B | Grin2b | Ionotropic glutamate receptor NMDA1/2B | N-methyl D-aspartate receptor subtype 2B | NMDAR2B | NMDE2_MOUSE | NR2B | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 165965.49 | ||
| Organism: | Mus musculus | ||
| Description: | ChEMBL_117725 | ||
| Residue: | 1482 | ||
| Sequence: |
| ||
| BDBM50269754 | |||
| n/a | |||
| Name | BDBM50269754 | ||
| Synonyms: | CHEMBL4077427 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H24N2O4 | ||
| Mol. Mass. | 404.4584 | ||
| SMILES | O[C@]1(Cc2cc(on2)-c2ccc3[nH]c(=O)oc3c2)CC[C@H](Cc2ccccc2)CC1 |r,wD:1.0,20.23,(15.39,-6.27,;16.16,-7.61,;14.62,-7.61,;13.85,-8.94,;12.32,-9.1,;12,-10.6,;13.33,-11.37,;14.48,-10.34,;10.59,-11.23,;10.44,-12.76,;9.04,-13.39,;7.79,-12.48,;6.28,-12.8,;5.51,-11.46,;3.98,-11.29,;6.54,-10.32,;7.95,-10.95,;9.35,-10.32,;17.49,-6.83,;18.82,-7.61,;18.82,-9.15,;20.15,-9.92,;21.49,-9.15,;22.82,-9.92,;24.15,-9.16,;24.16,-7.62,;22.81,-6.84,;21.48,-7.62,;17.49,-9.91,;16.16,-9.15,)| | ||
| Structure | 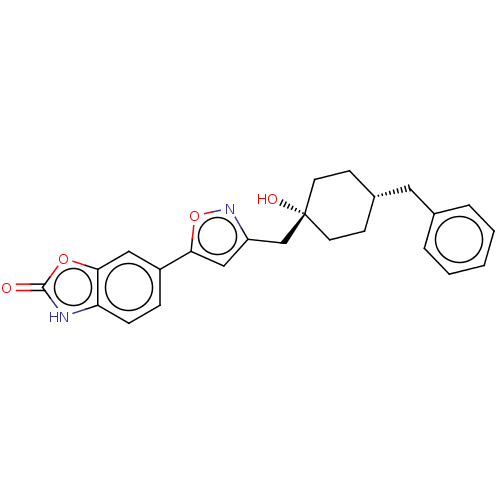
| ||