 Found 8103 hits with Last Name = 'hara' and Initial = 's'
Found 8103 hits with Last Name = 'hara' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157
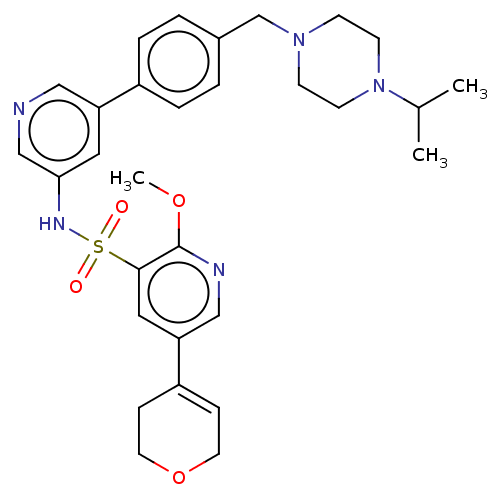
(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166
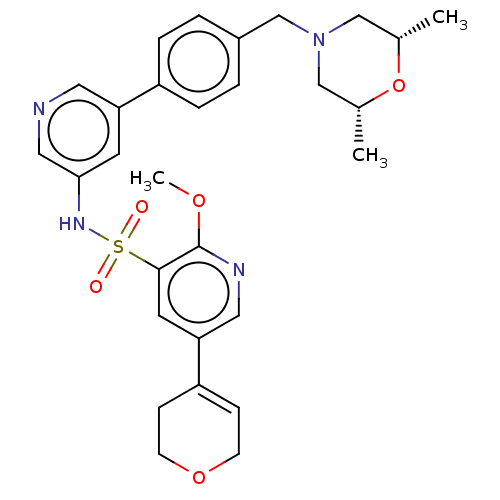
(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573177

(CHEMBL4167702)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573167
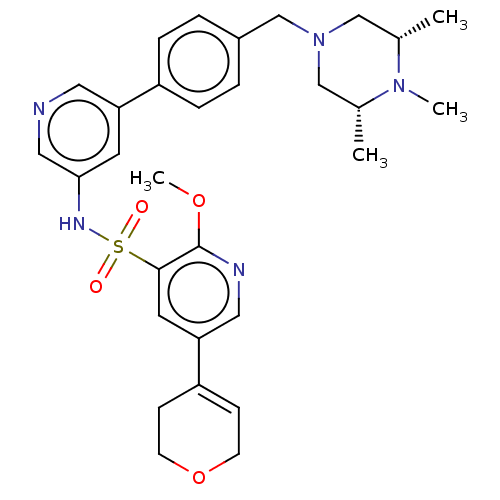
(CHEMBL4858875)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)N(C)[C@H](C)C2)cc1)C1=CCOCC1 |r,t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573164

(CHEMBL4873390)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)nc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573182
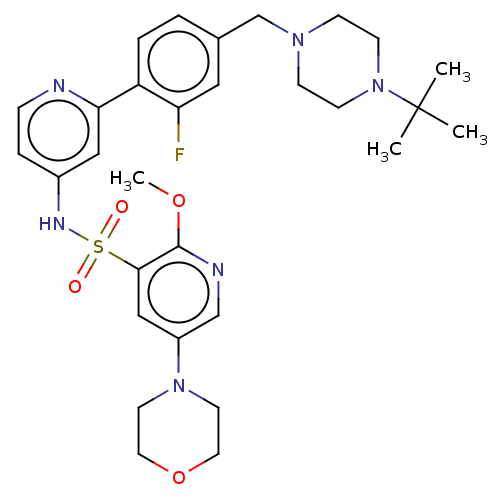
(CHEMBL4175571)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573181
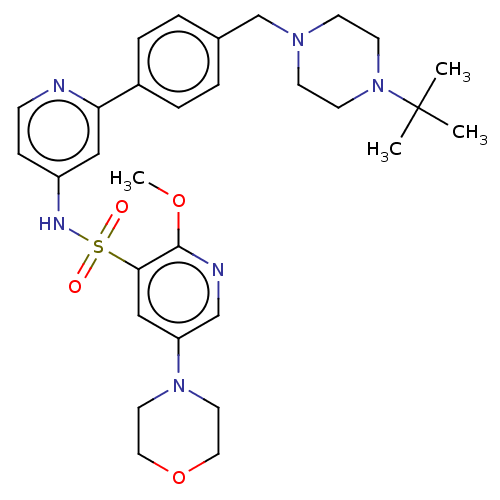
(CHEMBL4165185)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083556
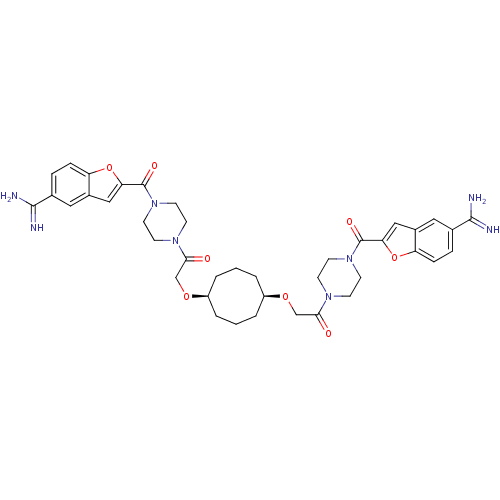
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573180
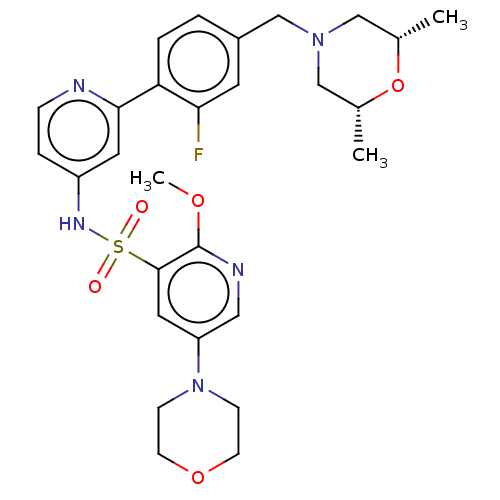
(CHEMBL4175737)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1F)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573175
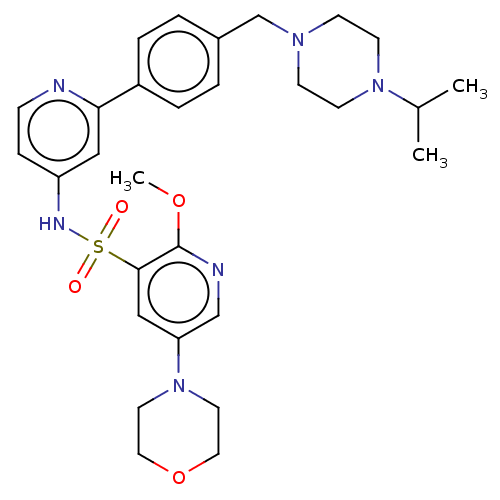
(CHEMBL4169192)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083541
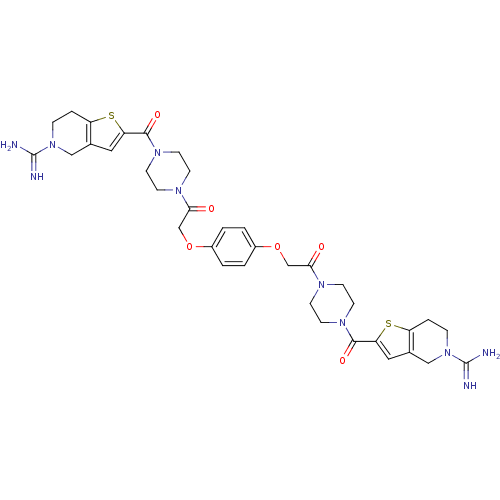
(1-{4-[5-amino(imino)methyl-4,5,6,7-tetrahydrothien...)Show SMILES NC(=N)N1CCc2sc(cc2C1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CN(CCc3s2)C(N)=N)cc1 Show InChI InChI=1S/C36H44N10O6S2/c37-35(38)45-7-5-27-23(19-45)17-29(53-27)33(49)43-13-9-41(10-14-43)31(47)21-51-25-1-2-26(4-3-25)52-22-32(48)42-11-15-44(16-12-42)34(50)30-18-24-20-46(36(39)40)8-6-28(24)54-30/h1-4,17-18H,5-16,19-22H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573179
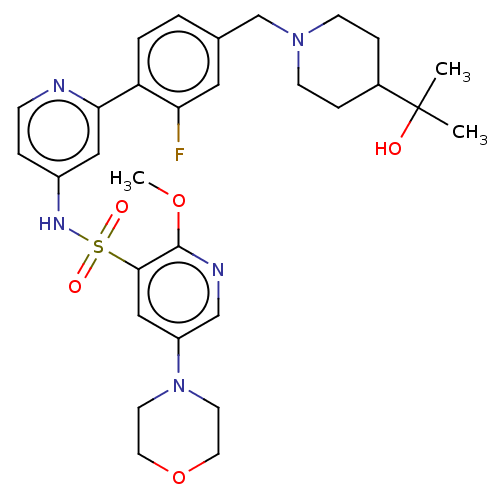
(CHEMBL4174874)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCC(CC2)C(C)(C)O)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573176

(CHEMBL4173087)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)c(F)c1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083548

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573178
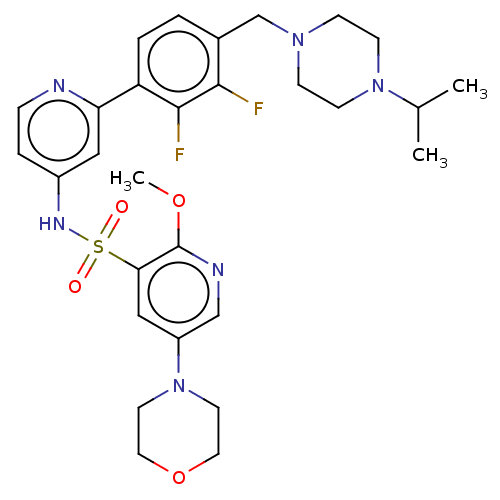
(CHEMBL4170075)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)c(F)c1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573170
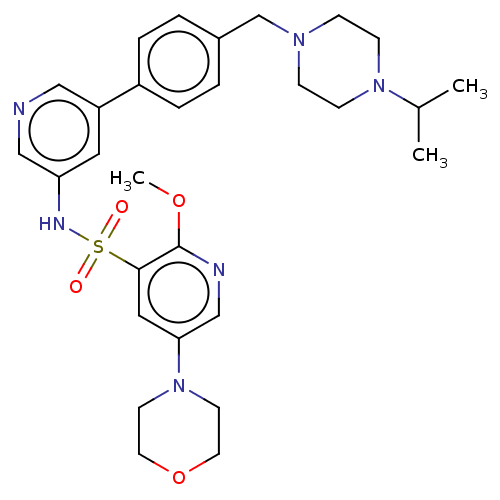
(CHEMBL4855234)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217306

(CHEMBL112049)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C36H42N8O6/c37-33(38)23-7-9-27-25(19-23)21-29(49-27)35(47)43-15-11-41(12-16-43)31(45)5-3-1-2-4-6-32(46)42-13-17-44(18-14-42)36(48)30-22-26-20-24(34(39)40)8-10-28(26)50-30/h7-10,19-22H,1-6,11-18H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573159
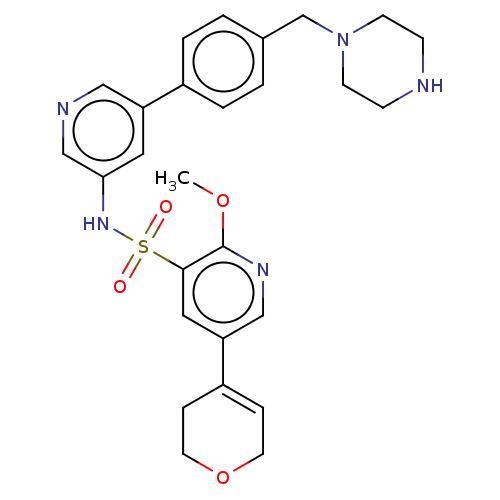
(CHEMBL4867203)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCNCC2)cc1)C1=CCOCC1 |t:35| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
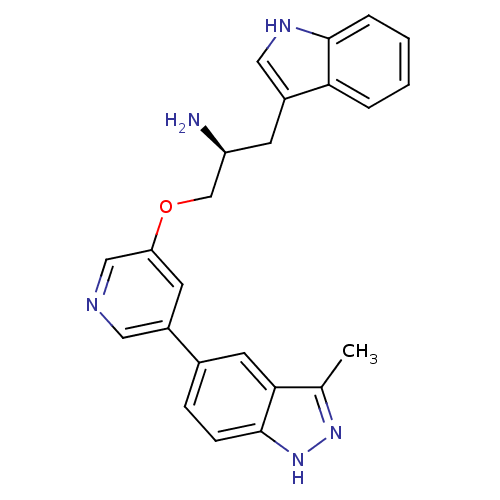
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01467
BindingDB Entry DOI: 10.7270/Q24Q801K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213889
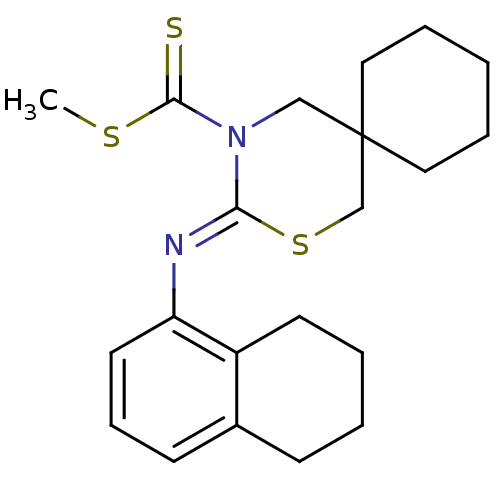
(3-[(Z)-5,6,7,8-tetrahydro-naphthalen-1-ylimino]-2-...)Show InChI InChI=1S/C21H28N2S3/c1-25-20(24)23-14-21(12-5-2-6-13-21)15-26-19(23)22-18-11-7-9-16-8-3-4-10-17(16)18/h7,9,11H,2-6,8,10,12-15H2,1H3/b22-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102398
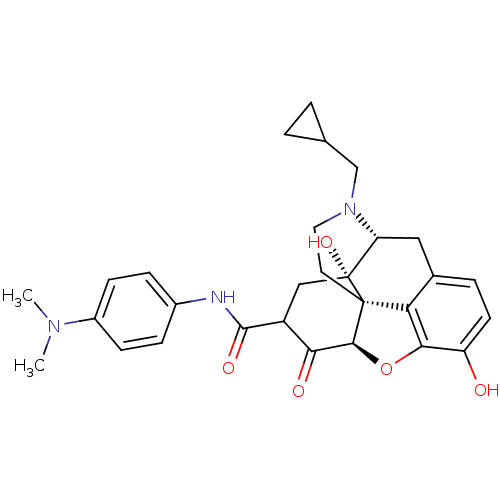
(US8536192, I-39)Show SMILES CN(C)c1ccc(NC(=O)C2C[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H](C2=O)[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:11:12:16.34.15:29.28.27,THB:13:12:16.34.15:29.28.27| Show InChI InChI=1S/C29H33N3O5/c1-31(2)19-8-6-18(7-9-19)30-27(35)20-14-29(36)22-13-17-5-10-21(33)25-23(17)28(29,26(37-25)24(20)34)11-12-32(22)15-16-3-4-16/h5-10,16,20,22,26,33,36H,3-4,11-15H2,1-2H3,(H,30,35)/t20?,22-,26+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102422
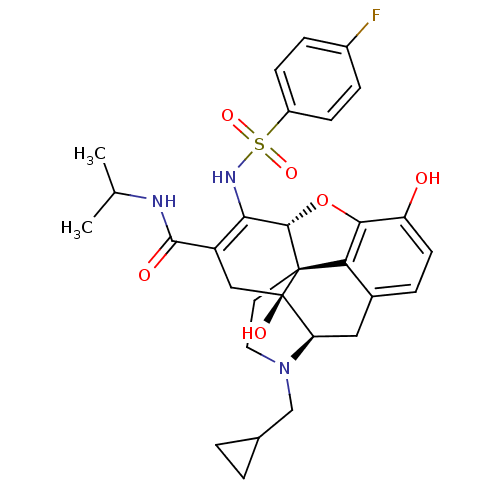
(US8536192, I-289)Show SMILES CC(C)NC(=O)C1=C(NS(=O)(=O)c2ccc(F)cc2)[C@@H]2Oc3c4c(C[C@H]5N(CC6CC6)CC[C@@]24[C@@]5(O)C1)ccc3O |r,c:6,TLB:35:34:22.23.24:32.26.31,THB:36:34:22.23.24:32.26.31| Show InChI InChI=1S/C30H34FN3O6S/c1-16(2)32-28(36)21-14-30(37)23-13-18-5-10-22(35)26-24(18)29(30,11-12-34(23)15-17-3-4-17)27(40-26)25(21)33-41(38,39)20-8-6-19(31)7-9-20/h5-10,16-17,23,27,33,35,37H,3-4,11-15H2,1-2H3,(H,32,36)/t23-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.470 | -53.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM82517
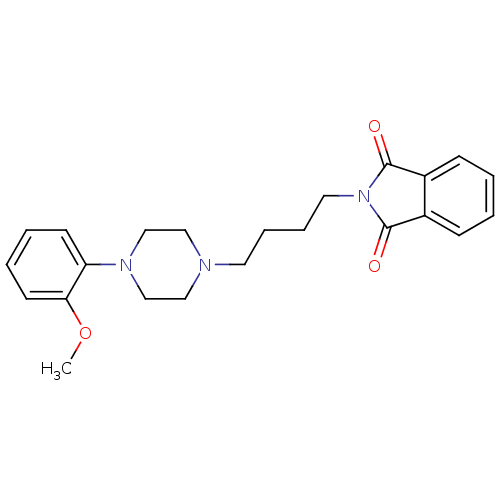
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Ability of the compound to displace [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor |
J Med Chem 42: 4952-60 (2000)
BindingDB Entry DOI: 10.7270/Q2833R7H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102415

(US8536192, I-244)Show SMILES CC(C)NC(=O)C1=C(NS(=O)(=O)c2ccc(Cl)cc2)[C@@H]2Oc3c4c(C[C@H]5N(CC6CC6)CC[C@@]24[C@@]5(O)C1)ccc3O |r,c:6,TLB:35:34:22.23.24:32.26.31,THB:36:34:22.23.24:32.26.31| Show InChI InChI=1S/C30H34ClN3O6S/c1-16(2)32-28(36)21-14-30(37)23-13-18-5-10-22(35)26-24(18)29(30,11-12-34(23)15-17-3-4-17)27(40-26)25(21)33-41(38,39)20-8-6-19(31)7-9-20/h5-10,16-17,23,27,33,35,37H,3-4,11-15H2,1-2H3,(H,32,36)/t23-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.560 | -52.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50503606
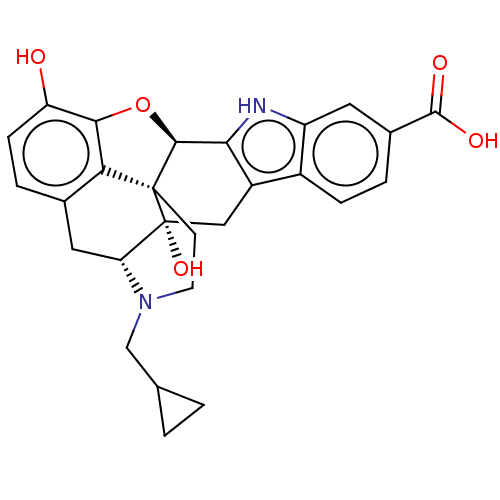
(CHEMBL4462756)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)Cc1c2[nH]c2cc(ccc12)C(O)=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26N2O5/c30-19-6-4-14-10-20-27(33)11-17-16-5-3-15(25(31)32)9-18(16)28-22(17)24-26(27,21(14)23(19)34-24)7-8-29(20)12-13-1-2-13/h3-6,9,13,20,24,28,30,33H,1-2,7-8,10-12H2,(H,31,32)/t20-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DADLE from recombinant human delta-opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 29: 73-77 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.007
BindingDB Entry DOI: 10.7270/Q2H41VQS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50213889

(3-[(Z)-5,6,7,8-tetrahydro-naphthalen-1-ylimino]-2-...)Show InChI InChI=1S/C21H28N2S3/c1-25-20(24)23-14-21(12-5-2-6-13-21)15-26-19(23)22-18-11-7-9-16-8-3-4-10-17(16)18/h7,9,11H,2-6,8,10,12-15H2,1H3/b22-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to mouse CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50256379
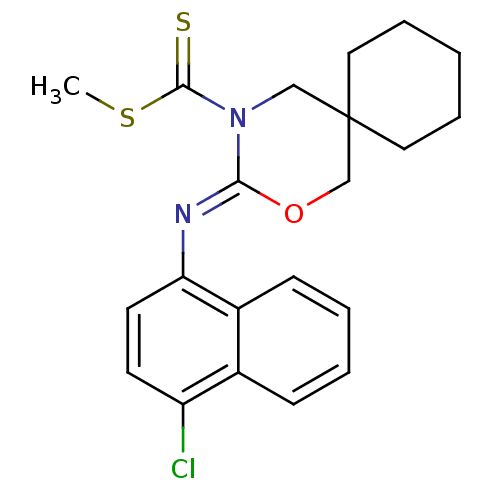
(CHEMBL482356 | methyl 3-(4-chloronaphthalen-1-ylim...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1ccc(Cl)c2ccccc12 Show InChI InChI=1S/C21H23ClN2OS2/c1-27-20(26)24-13-21(11-5-2-6-12-21)14-25-19(24)23-18-10-9-17(22)15-7-3-4-8-16(15)18/h3-4,7-10H,2,5-6,11-14H2,1H3/b23-19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to mouse CB1 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256378
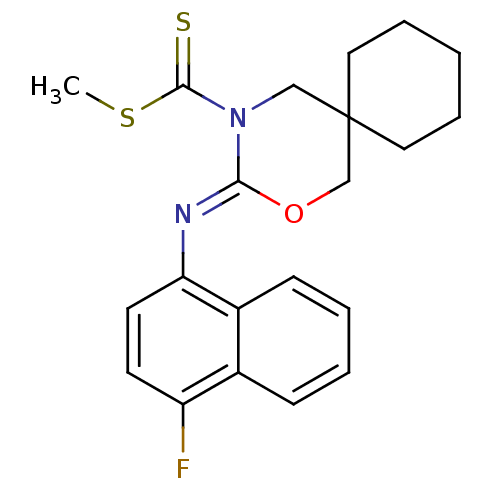
(CHEMBL482355 | methyl 3-(4-fluoronaphthalen-1-ylim...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1ccc(F)c2ccccc12 Show InChI InChI=1S/C21H23FN2OS2/c1-27-20(26)24-13-21(11-5-2-6-12-21)14-25-19(24)23-18-10-9-17(22)15-7-3-4-8-16(15)18/h3-4,7-10H,2,5-6,11-14H2,1H3/b23-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213881

(3-[(Z)-2-isopropyl-phenylimino]-2-thia-4-aza-spiro...)Show InChI InChI=1S/C20H28N2S3/c1-15(2)16-9-5-6-10-17(16)21-18-22(19(23)24-3)13-20(14-25-18)11-7-4-8-12-20/h5-6,9-10,15H,4,7-8,11-14H2,1-3H3/b21-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102416

(US8536192, I-267)Show SMILES Cc1ccc2nc(=O)c3C[C@@]4(O)[C@H]5Cc6ccc(O)c7O[C@@H](c3n2c1)[C@]4(CCN5CC1CC1)c67 |r,TLB:9:10:33.14.13:26.28.27,THB:11:10:33.14.13:26.28.27| Show InChI InChI=1S/C27H27N3O4/c1-14-2-7-20-28-25(32)17-11-27(33)19-10-16-5-6-18(31)23-21(16)26(27,8-9-29(19)13-15-3-4-15)24(34-23)22(17)30(20)12-14/h2,5-7,12,15,19,24,31,33H,3-4,8-11,13H2,1H3/t19-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.840 | -51.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213900
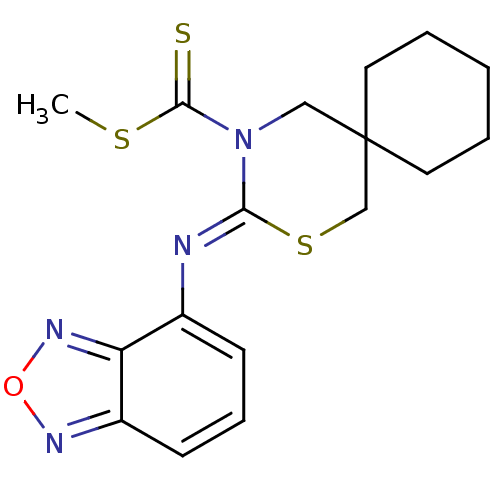
(3-[(Z)-benzo[1,2,5]oxadiazol-4-ylimino]-2-thia-4-a...)Show InChI InChI=1S/C17H20N4OS3/c1-24-16(23)21-10-17(8-3-2-4-9-17)11-25-15(21)18-12-6-5-7-13-14(12)20-22-19-13/h5-7H,2-4,8-11H2,1H3/b18-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256379

(CHEMBL482356 | methyl 3-(4-chloronaphthalen-1-ylim...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1ccc(Cl)c2ccccc12 Show InChI InChI=1S/C21H23ClN2OS2/c1-27-20(26)24-13-21(11-5-2-6-12-21)14-25-19(24)23-18-10-9-17(22)15-7-3-4-8-16(15)18/h3-4,7-10H,2,5-6,11-14H2,1H3/b23-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256315
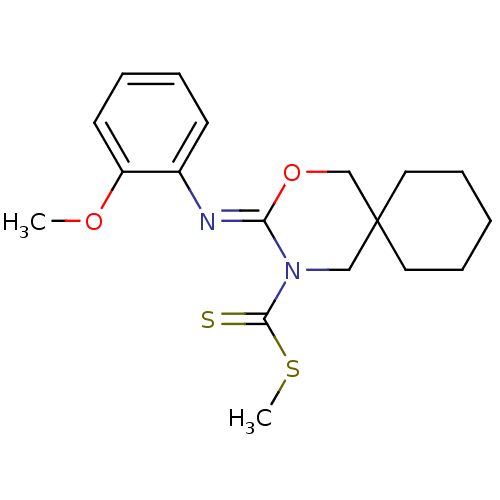
(CHEMBL469895 | methyl 3-(2-methoxyphenylimino)-2-o...)Show InChI InChI=1S/C18H24N2O2S2/c1-21-15-9-5-4-8-14(15)19-16-20(17(23)24-2)12-18(13-22-16)10-6-3-7-11-18/h4-5,8-9H,3,6-7,10-13H2,1-2H3/b19-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256218
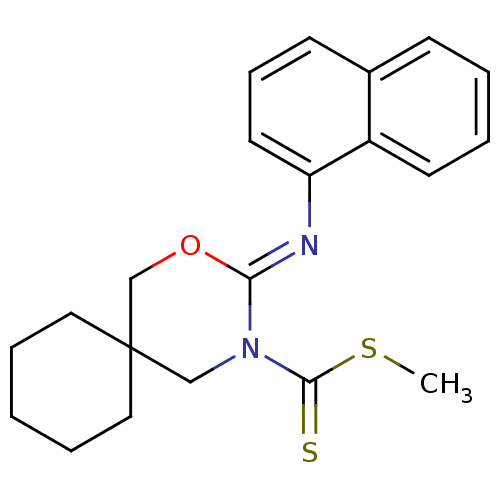
(CHEMBL516406 | methyl 3-(naphthalen-1-ylimino)-2-o...)Show InChI InChI=1S/C21H24N2OS2/c1-26-20(25)23-14-21(12-5-2-6-13-21)15-24-19(23)22-18-11-7-9-16-8-3-4-10-17(16)18/h3-4,7-11H,2,5-6,12-15H2,1H3/b22-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50256318
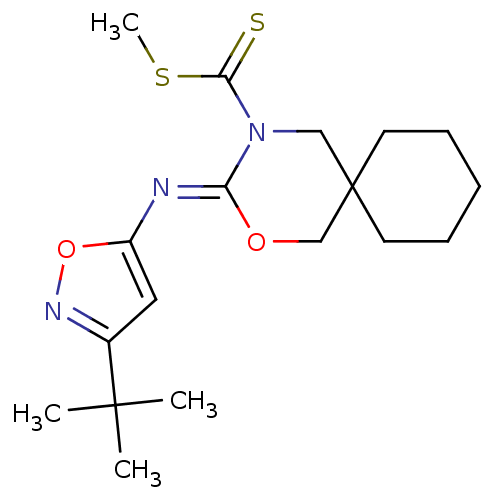
(CHEMBL481508 | methyl 3-(3-tert-butylisoxazol-5-yl...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1cc(no1)C(C)(C)C Show InChI InChI=1S/C18H27N3O2S2/c1-17(2,3)13-10-14(23-20-13)19-15-21(16(24)25-4)11-18(12-22-15)8-6-5-7-9-18/h10H,5-9,11-12H2,1-4H3/b19-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM403740

(6-Chloro-7-((3-(cyclopropylmethyl)-7-methyl-3H-imi...)Show SMILES CN1C(=O)COc2cc(Nc3cc(C)c4ncn(CC5CC5)c4n3)c(Cl)cc12 Show InChI InChI=1S/C20H20ClN5O2/c1-11-5-17(24-20-19(11)22-10-26(20)8-12-3-4-12)23-14-7-16-15(6-13(14)21)25(2)18(27)9-28-16/h5-7,10,12H,3-4,8-9H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
US Patent
| Assay Description
The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... |
US Patent US10016439 (2018)
BindingDB Entry DOI: 10.7270/Q2NC63J0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50503604
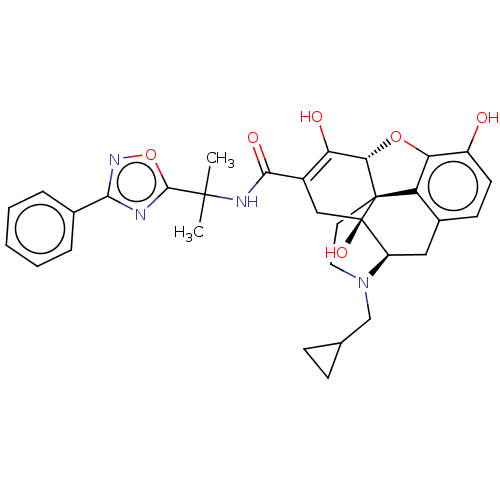
(Naldemedine | S-297995)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC(C(=O)NC(C)(C)c1nc(no1)-c1ccccc1)=C2O)ccc3O |r,c:43| Show InChI InChI=1S/C32H34N4O6/c1-30(2,29-33-27(35-42-29)18-6-4-3-5-7-18)34-28(39)20-15-32(40)22-14-19-10-11-21(37)25-23(19)31(32,26(41-25)24(20)38)12-13-36(22)16-17-8-9-17/h3-7,10-11,17,22,26,37-38,40H,8-9,12-16H2,1-2H3,(H,34,39)/t22-,26+,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DADLE from recombinant human delta-opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 29: 73-77 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.007
BindingDB Entry DOI: 10.7270/Q2H41VQS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102418
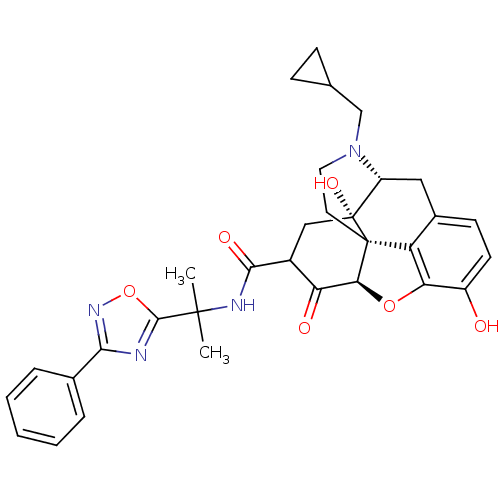
(US8536192, I-284)Show SMILES CC(C)(NC(=O)C1C[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H](C1=O)[C@]2(CCN3CC1CC1)c45)c1nc(no1)-c1ccccc1 |r,TLB:7:8:30.12.11:23.25.24,THB:9:8:30.12.11:23.25.24| Show InChI InChI=1S/C32H34N4O6/c1-30(2,29-33-27(35-42-29)18-6-4-3-5-7-18)34-28(39)20-15-32(40)22-14-19-10-11-21(37)25-23(19)31(32,26(41-25)24(20)38)12-13-36(22)16-17-8-9-17/h3-7,10-11,17,20,22,26,37,40H,8-9,12-16H2,1-2H3,(H,34,39)/t20?,22-,26+,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.910 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50503597
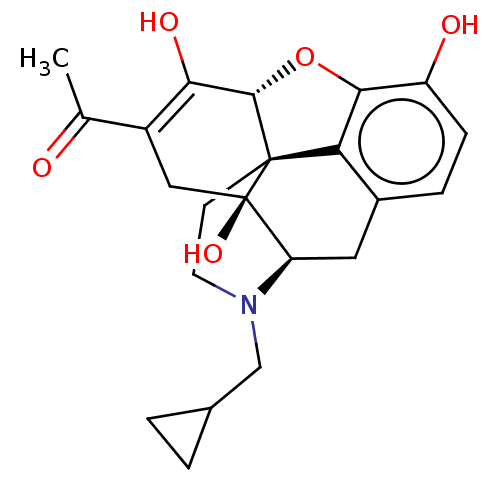
(CHEMBL4534307)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC(C(C)=O)=C2O)ccc3O |r,c:27,THB:10:9:17:4.5.6| Show InChI InChI=1S/C22H25NO5/c1-11(24)14-9-22(27)16-8-13-4-5-15(25)19-17(13)21(22,20(28-19)18(14)26)6-7-23(16)10-12-2-3-12/h4-5,12,16,20,25-27H,2-3,6-10H2,1H3/t16-,20+,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from recombinant human mu-opioid receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 29: 73-77 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.007
BindingDB Entry DOI: 10.7270/Q2H41VQS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50256317
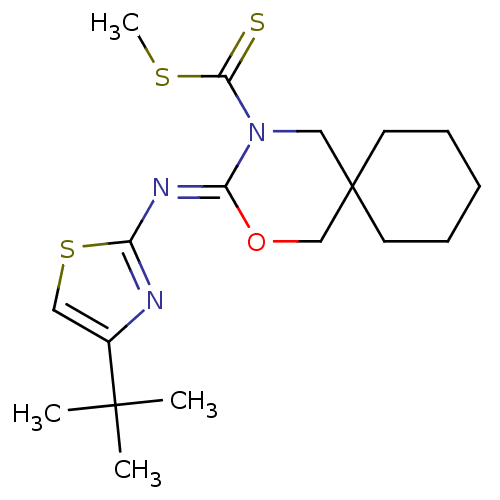
(CHEMBL511790 | methyl 3-(4-tert-butylthiazol-2-yli...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1nc(cs1)C(C)(C)C Show InChI InChI=1S/C18H27N3OS3/c1-17(2,3)13-10-25-14(19-13)20-15-21(16(23)24-4)11-18(12-22-15)8-6-5-7-9-18/h10H,5-9,11-12H2,1-4H3/b20-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256431
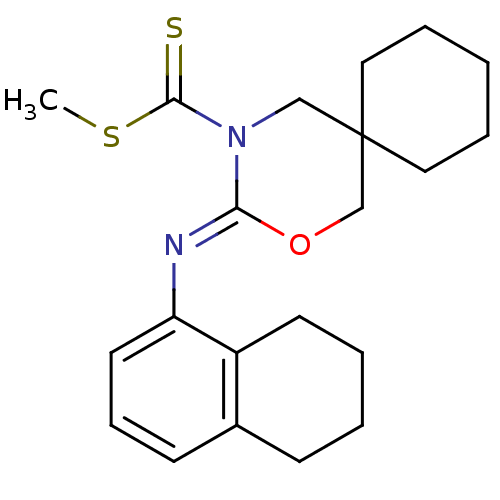
(CHEMBL480578 | methyl 3-(5,6,7,8-tetrahydronaphtha...)Show InChI InChI=1S/C21H28N2OS2/c1-26-20(25)23-14-21(12-5-2-6-13-21)15-24-19(23)22-18-11-7-9-16-8-3-4-10-17(16)18/h7,9,11H,2-6,8,10,12-15H2,1H3/b22-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213891
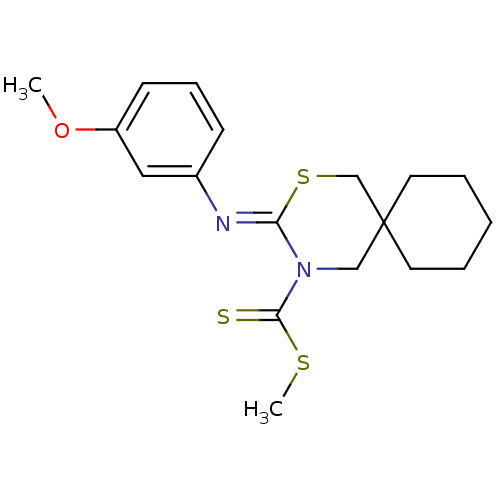
(3-[(Z)-3-methoxy-phenylimino]-2-thia-4-aza-spiro[5...)Show InChI InChI=1S/C18H24N2OS3/c1-21-15-8-6-7-14(11-15)19-16-20(17(22)23-2)12-18(13-24-16)9-4-3-5-10-18/h6-8,11H,3-5,9-10,12-13H2,1-2H3/b19-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256319
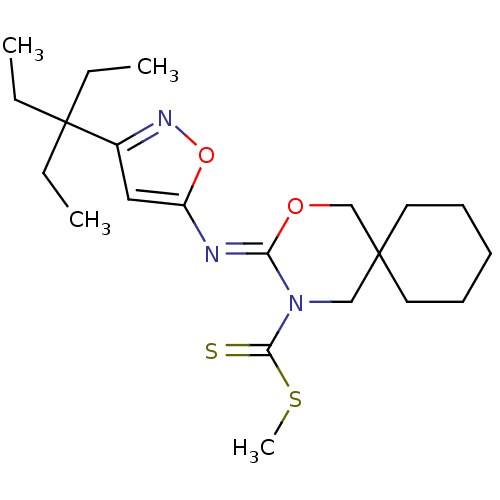
(CHEMBL519288 | methyl 3-(3-(3-ethylpentan-3-yl)iso...)Show SMILES CCC(CC)(CC)c1cc(\N=C2/OCC3(CCCCC3)CN2C(=S)SC)on1 Show InChI InChI=1S/C21H33N3O2S2/c1-5-21(6-2,7-3)16-13-17(26-23-16)22-18-24(19(27)28-4)14-20(15-25-18)11-9-8-10-12-20/h13H,5-12,14-15H2,1-4H3/b22-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50256318

(CHEMBL481508 | methyl 3-(3-tert-butylisoxazol-5-yl...)Show SMILES CSC(=S)N1CC2(CCCCC2)CO\C1=N/c1cc(no1)C(C)(C)C Show InChI InChI=1S/C18H27N3O2S2/c1-17(2,3)13-10-14(23-20-13)19-15-21(16(24)25-4)11-18(12-22-15)8-6-5-7-9-18/h10H,5-9,11-12H2,1-4H3/b19-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50503611
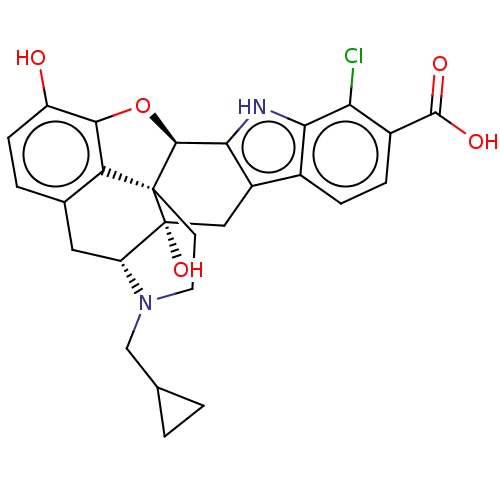
(CHEMBL4468216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)Cc1c2[nH]c2c(Cl)c(ccc12)C(O)=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H25ClN2O5/c28-20-15(25(32)33)5-4-14-16-10-27(34)18-9-13-3-6-17(31)23-19(13)26(27,7-8-30(18)11-12-1-2-12)24(35-23)22(16)29-21(14)20/h3-6,12,18,24,29,31,34H,1-2,7-11H2,(H,32,33)/t18-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DADLE from recombinant human delta-opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 29: 73-77 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.007
BindingDB Entry DOI: 10.7270/Q2H41VQS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213883
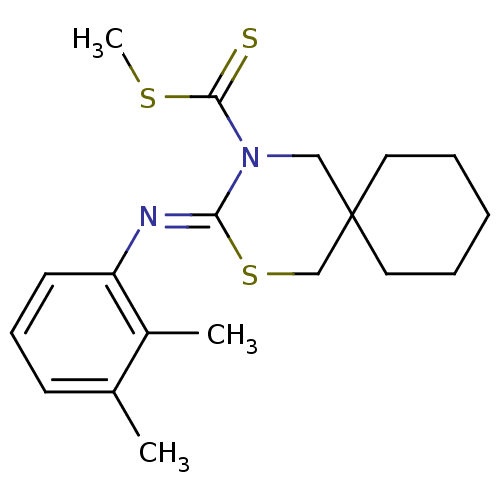
(3-[(Z)-2,3-dimethyl-phenylimino]-2-thia-4-aza-spir...)Show InChI InChI=1S/C19H26N2S3/c1-14-8-7-9-16(15(14)2)20-17-21(18(22)23-3)12-19(13-24-17)10-5-4-6-11-19/h7-9H,4-6,10-13H2,1-3H3/b20-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213888
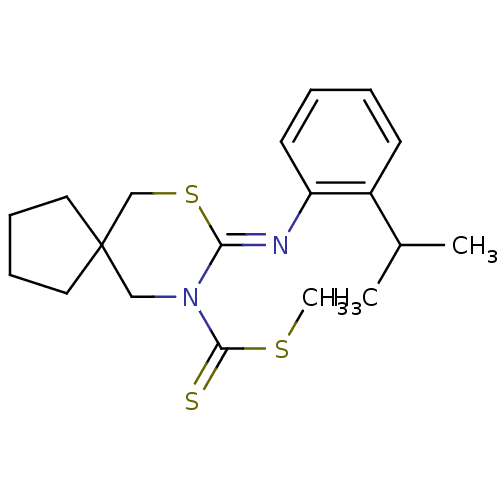
(8-[(Z)-2-isopropyl-phenylimino]-7-thia-9-aza-spiro...)Show InChI InChI=1S/C19H26N2S3/c1-14(2)15-8-4-5-9-16(15)20-17-21(18(22)23-3)12-19(13-24-17)10-6-7-11-19/h4-5,8-9,14H,6-7,10-13H2,1-3H3/b20-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 3925-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.099
BindingDB Entry DOI: 10.7270/Q2RX9BR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50256319

(CHEMBL519288 | methyl 3-(3-(3-ethylpentan-3-yl)iso...)Show SMILES CCC(CC)(CC)c1cc(\N=C2/OCC3(CCCCC3)CN2C(=S)SC)on1 Show InChI InChI=1S/C21H33N3O2S2/c1-5-21(6-2,7-3)16-13-17(26-23-16)22-18-24(19(27)28-4)14-20(15-25-18)11-9-8-10-12-20/h13H,5-12,14-15H2,1-4H3/b22-18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 18: 6444-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.070
BindingDB Entry DOI: 10.7270/Q20V8CNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data