| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cysteinyl leukotriene receptor 1 |
|---|
| Ligand | BDBM50013539 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_52212 |
|---|
| Ki | 170±n/a nM |
|---|
| Citation |  Youssefyeh, RD; Magnien, E; Lee, TD; Chan, WK; Lin, CJ; Galemmo, RA; Johnson, WH; Tan, J; Campbell, HF; Huang, FC Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists. 1. Initial structure-activity relationships. J Med Chem33:1186-94 (1990) [PubMed] Youssefyeh, RD; Magnien, E; Lee, TD; Chan, WK; Lin, CJ; Galemmo, RA; Johnson, WH; Tan, J; Campbell, HF; Huang, FC Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists. 1. Initial structure-activity relationships. J Med Chem33:1186-94 (1990) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cysteinyl leukotriene receptor 1 |
|---|
| Name: | Cysteinyl leukotriene receptor 1 |
|---|
| Synonyms: | CLTR1_CAVPO | CYSLTR1 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 39007.58 |
|---|
| Organism: | GUINEA PIG |
|---|
| Description: | Leukotriene D4 0 GUINEA PIG::Q2NNR5 |
|---|
| Residue: | 340 |
|---|
| Sequence: | MDETGNPTIPPASNNTCYDSIDDFRNQVYSTLYSMISVVGFFGNGFVLYVLVKTYHEKSA
FQVYMINLAVADLLCVCTLPLRVAYYVHKGIWLFGDFLCRLSTYALYVNLYCSIFFMTAM
SFFRCVAIVFPVQNISLVTQKKARLVCIAIWMFVILTSSPFLMANTYKDEKNNTKCFEPP
QDNQAKNYVLILHYVSLFIGFIIPFITIIVCYTMIIFTLLKSSMKKNLSSRKRAIGMIIV
VTAAFLVSFMPYHIQRTIHLHFLHNKTKPCDSILRMQKSVVITLSLAASNCCFDPLLYFF
SGGNFRRRLSTIRKYSLSSMTYIPKKKTSLPQKGKDICKE
|
|
|
|---|
| BDBM50013539 |
|---|
| n/a |
|---|
| Name | BDBM50013539 |
|---|
| Synonyms: | 2-{3-Methyl-4-[3-(1H-tetrazol-5-yl)-propoxy]-phenoxymethyl}-quinoline | CHEMBL406006 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21N5O2 |
|---|
| Mol. Mass. | 375.4237 |
|---|
| SMILES | Cc1cc(OCc2ccc3ccccc3n2)ccc1OCCCc1nnn[nH]1 |
|---|
| Structure | 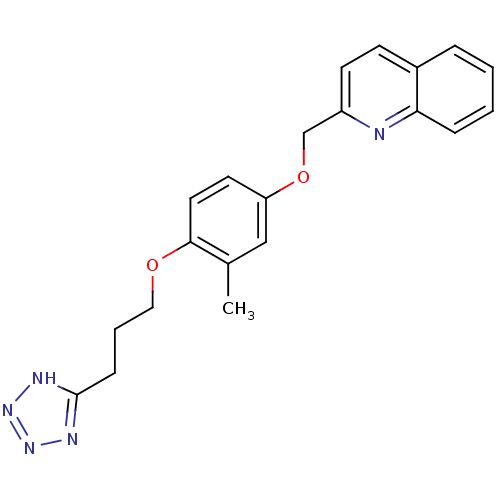
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Youssefyeh, RD; Magnien, E; Lee, TD; Chan, WK; Lin, CJ; Galemmo, RA; Johnson, WH; Tan, J; Campbell, HF; Huang, FC Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists. 1. Initial structure-activity relationships. J Med Chem33:1186-94 (1990) [PubMed]
Youssefyeh, RD; Magnien, E; Lee, TD; Chan, WK; Lin, CJ; Galemmo, RA; Johnson, WH; Tan, J; Campbell, HF; Huang, FC Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists. 1. Initial structure-activity relationships. J Med Chem33:1186-94 (1990) [PubMed]