

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097624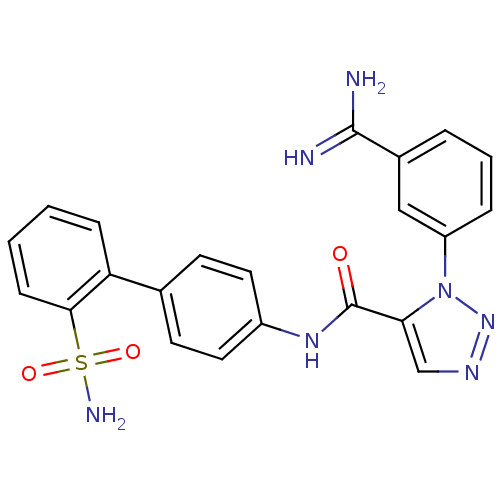 (3-(3-Carbamimidoyl-phenyl)-3H-[1,2,3]triazole-4-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693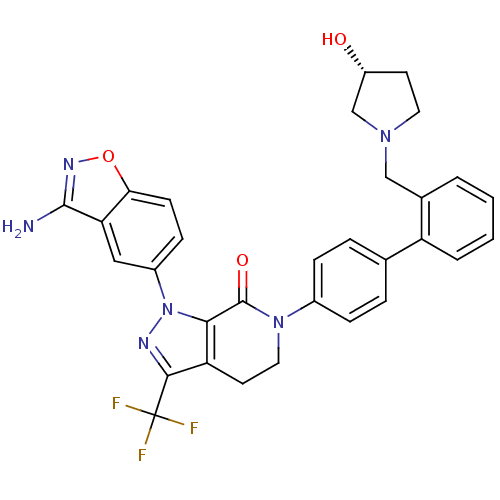 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human trypsin | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097626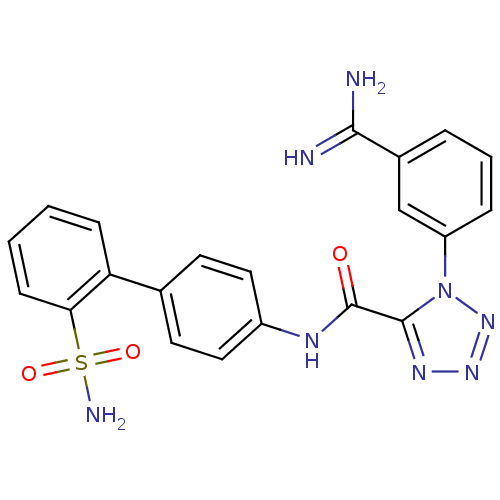 (1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681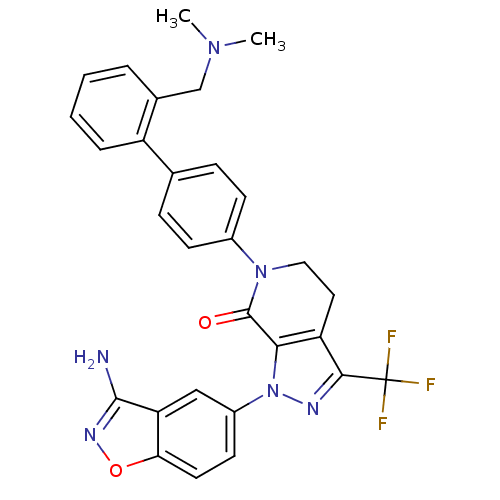 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288406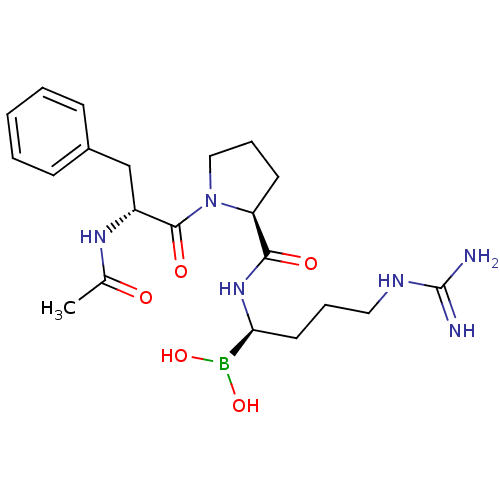 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of trypsin | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097625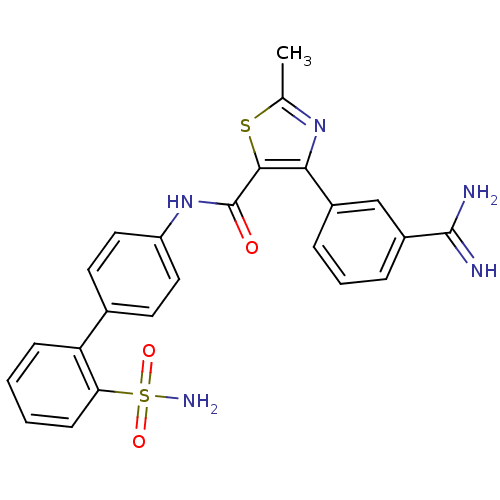 (4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50097625 (4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human trypsin | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12690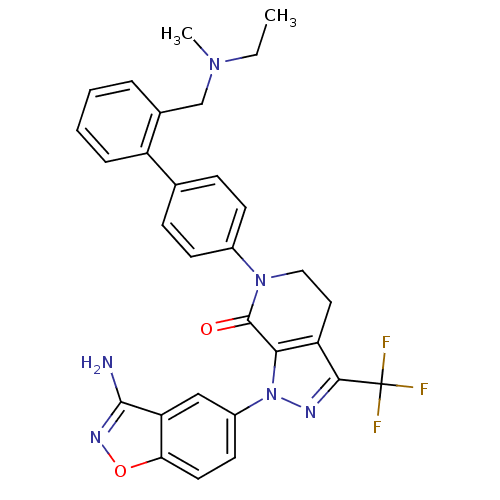 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[ethyl(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | <-56.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291847 (7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12689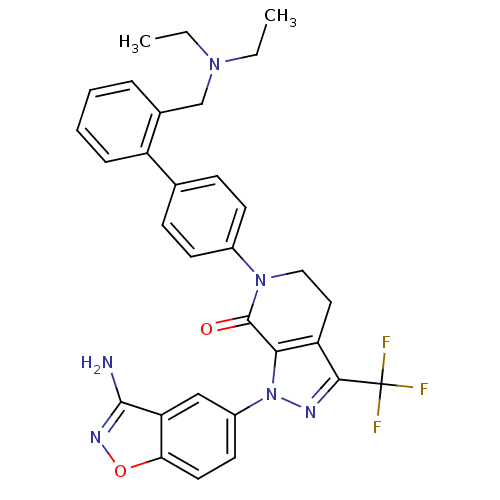 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(diethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12694 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291855 (7-[3-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12748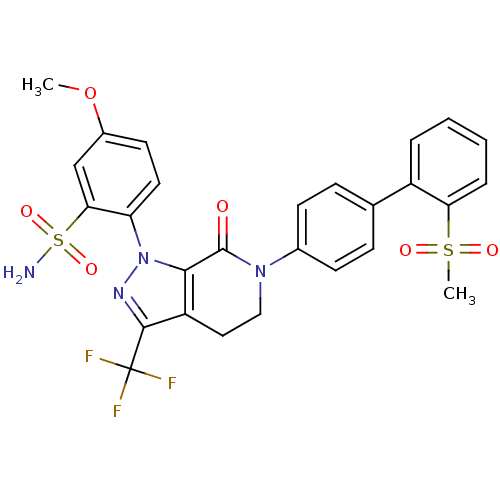 (2-{6-[4-(2-methanesulfonylphenyl)phenyl]-7-oxo-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12682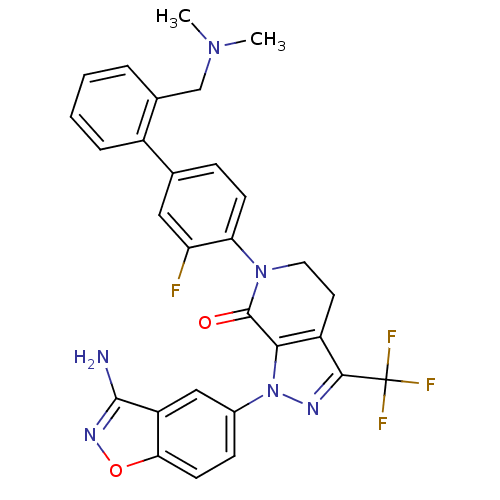 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657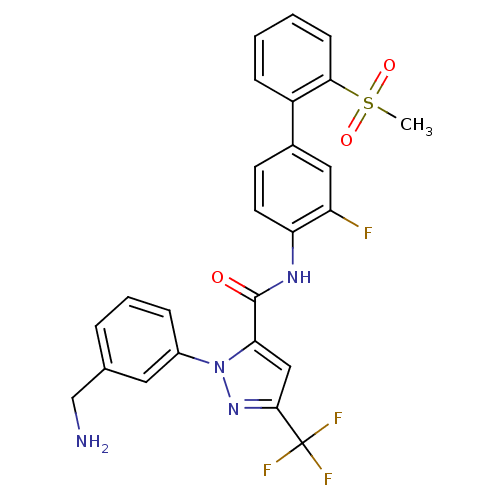 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12692 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087533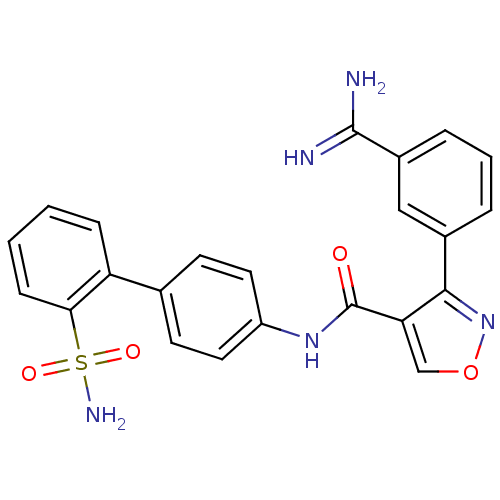 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288405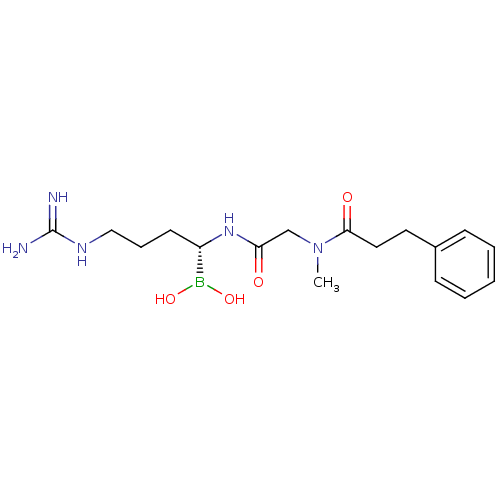 (CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096095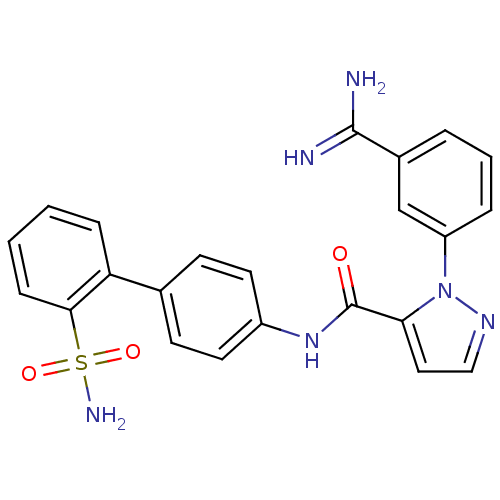 (2-(3-Carbamimidoyl-phenyl)-2H-pyrazole-3-carboxyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12700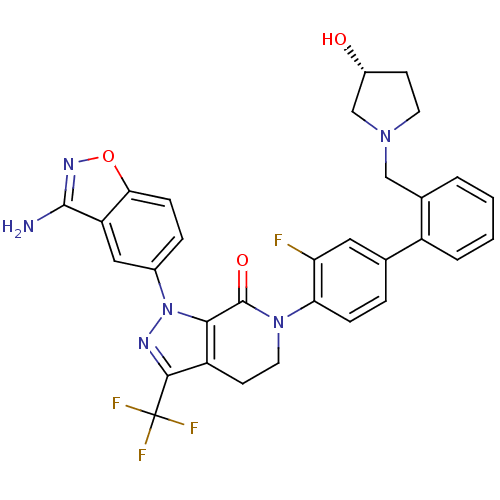 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12746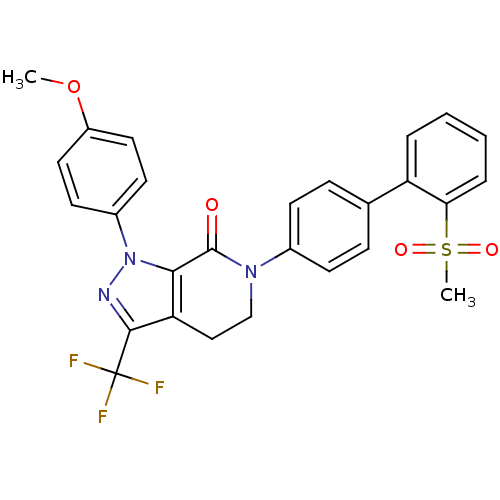 (6-[4-(2-methanesulfonylphenyl)phenyl]-1-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12747 (2-{6-[4-(2-methanesulfonylphenyl)phenyl]-7-oxo-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12733 (3-[6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097620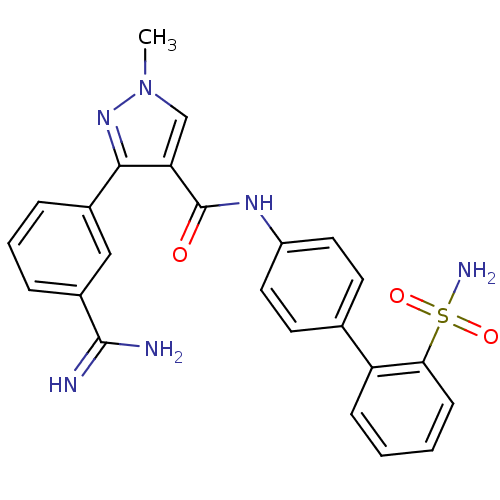 (3-(3-Carbamimidoyl-phenyl)-1-methyl-1H-pyrazole-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50009075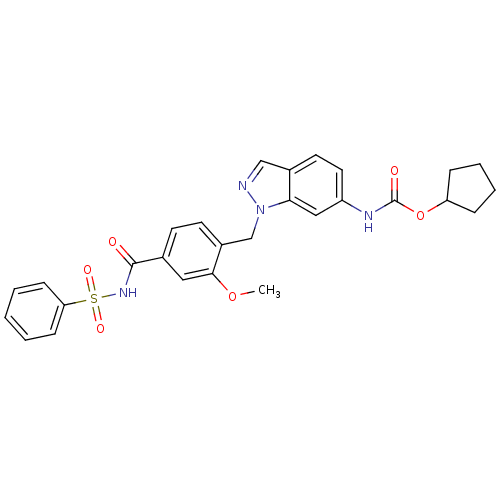 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro binding affinity against cysteinyl leukotriene D4 receptor from guinea pig lung membrane | J Med Chem 33: 2828-41 (1990) BindingDB Entry DOI: 10.7270/Q29C6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12749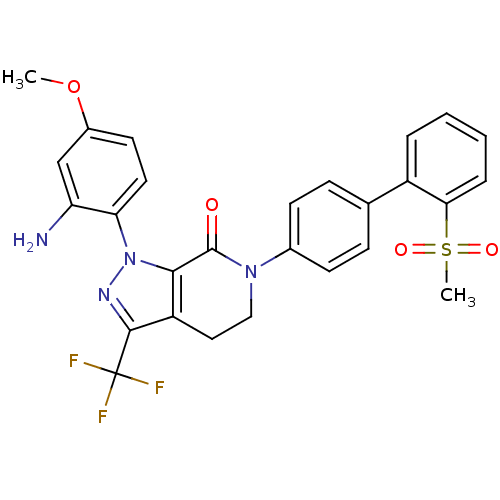 (1-(2-amino-4-methoxyphenyl)-6-[4-(2-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12695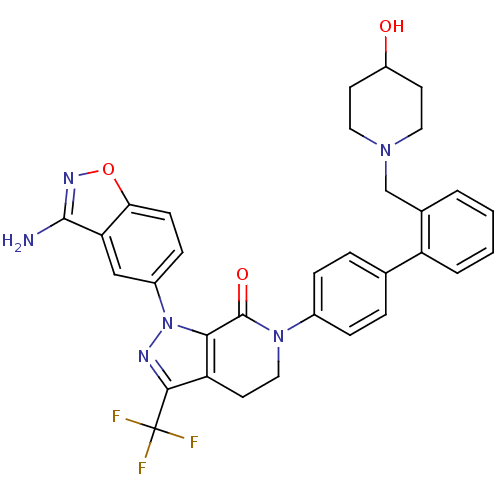 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288414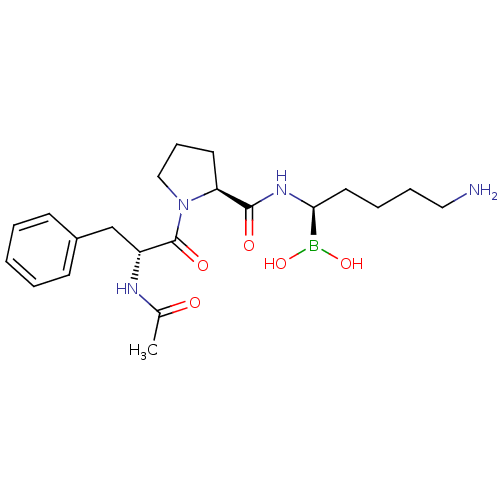 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12699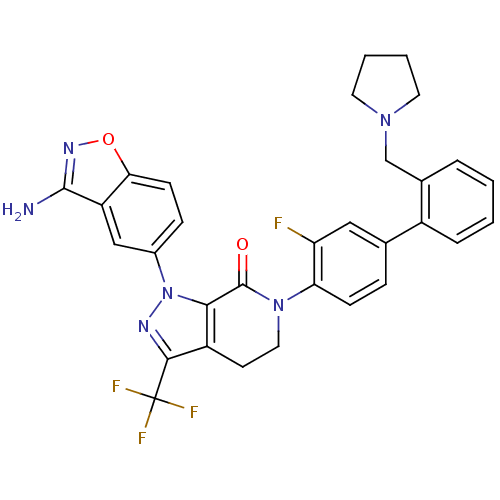 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{2-fluoro-4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12687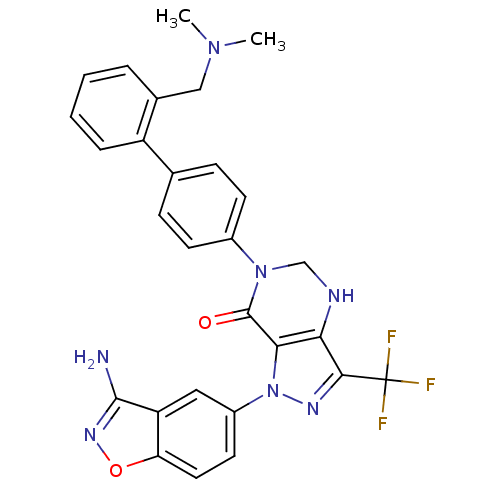 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12688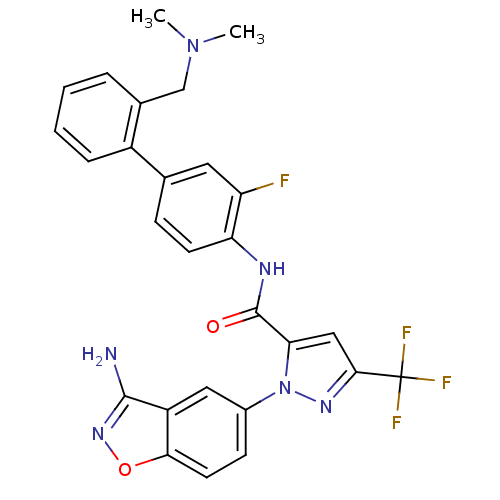 (1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19042 (6-(1-{2-[(diethylamino)methyl]phenyl}piperidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 17: 1432-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.071 BindingDB Entry DOI: 10.7270/Q2KD1W5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19038 (1-(4-methoxyphenyl)-7-oxo-6-{1-[2-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 17: 1432-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.071 BindingDB Entry DOI: 10.7270/Q2KD1W5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12703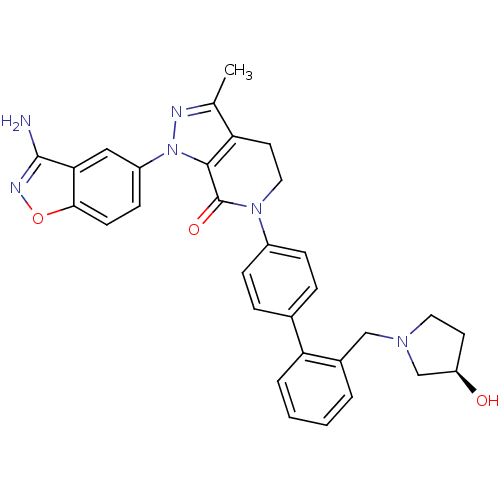 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009075 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291848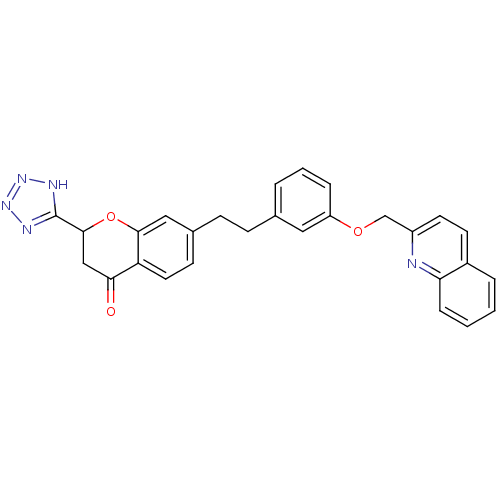 (7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-ethyl}-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 17: 1432-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.071 BindingDB Entry DOI: 10.7270/Q2KD1W5D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097619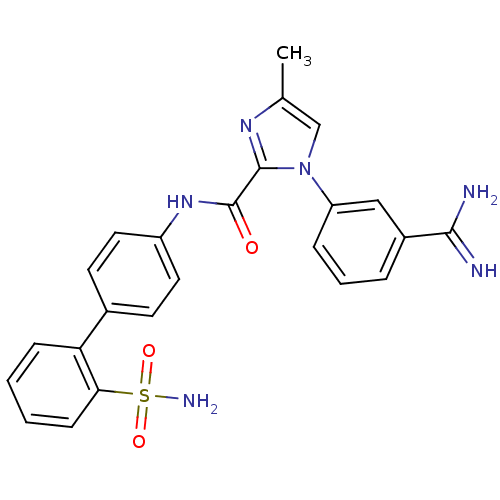 (1-(3-Carbamimidoyl-phenyl)-4-methyl-1H-imidazole-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human Serine protease FXa | Bioorg Med Chem Lett 11: 641-5 (2001) BindingDB Entry DOI: 10.7270/Q2RV0MZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of rabbit Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12702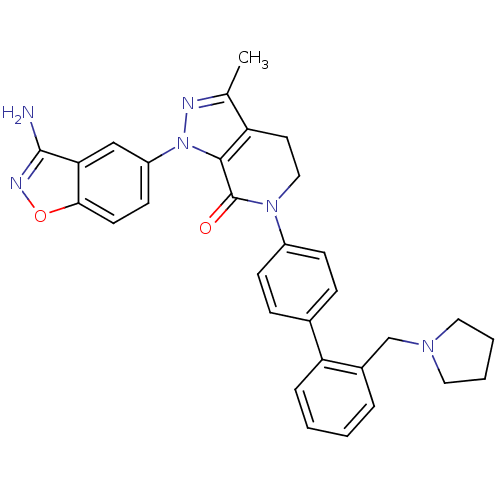 (1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50094092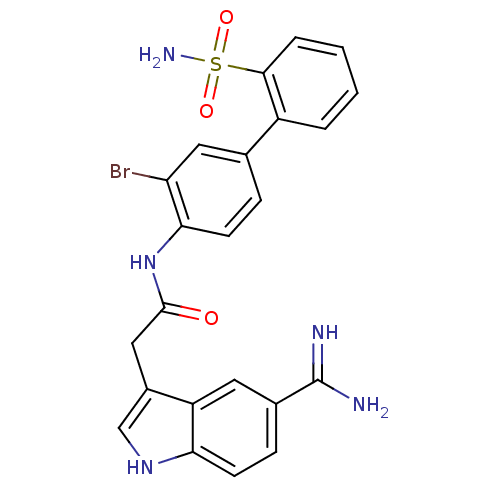 (CHEMBL336661 | N-(3-Bromo-2'-sulfamoyl-biphenyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 43: 4398-415 (2000) BindingDB Entry DOI: 10.7270/Q2JS9PP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1976 total ) | Next | Last >> |