| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prothrombin |
|---|
| Ligand | BDBM50288405 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_208357 |
|---|
| Ki | 0.160000±n/a nM |
|---|
| Citation |  Galemmo, RA; Fevig, JM; Carini, DJ; Cacciola, J; Wells, BL; Hillyer, GL; Jr., JB; Rossi, KA; Stouten, PF; Alexander, RS; Hilmer, R; Bostrom, L; Abelman, MM; Lee, SL; Weber, PC; Kettner, CA; Knabb, RM; Wexler, RR (N-acyl-N-alkyl)glycyl borolysine analogs: A new class of potent thrombin inhibitors Bioorg Med Chem Lett6:2913-2918 (1996) Article Galemmo, RA; Fevig, JM; Carini, DJ; Cacciola, J; Wells, BL; Hillyer, GL; Jr., JB; Rossi, KA; Stouten, PF; Alexander, RS; Hilmer, R; Bostrom, L; Abelman, MM; Lee, SL; Weber, PC; Kettner, CA; Knabb, RM; Wexler, RR (N-acyl-N-alkyl)glycyl borolysine analogs: A new class of potent thrombin inhibitors Bioorg Med Chem Lett6:2913-2918 (1996) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prothrombin |
|---|
| Name: | Prothrombin |
|---|
| Synonyms: | Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 70029.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00734 |
|---|
| Residue: | 622 |
|---|
| Sequence: | MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLEREC
VEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHV
NITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQE
CSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASA
QAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETG
DGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYI
DGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTEN
DLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHP
VCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDST
RIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKY
GFYTHVFRLKKWIQKVIDQFGE
|
|
|
|---|
| BDBM50288405 |
|---|
| n/a |
|---|
| Name | BDBM50288405 |
|---|
| Synonyms: | CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-butylcarbamoyl)-methyl]-N-methyl-3-phenyl-propionamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H28BN5O4 |
|---|
| Mol. Mass. | 377.246 |
|---|
| SMILES | CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 |
|---|
| Structure | 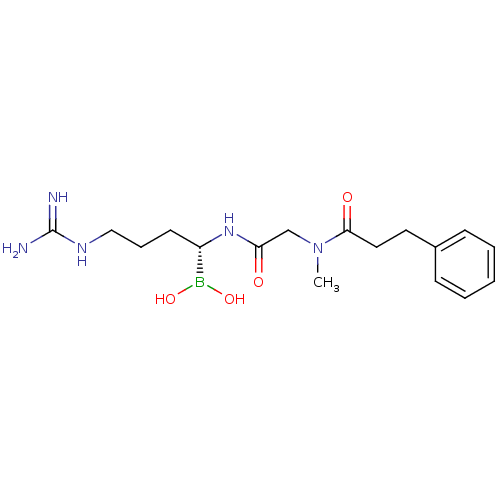
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Galemmo, RA; Fevig, JM; Carini, DJ; Cacciola, J; Wells, BL; Hillyer, GL; Jr., JB; Rossi, KA; Stouten, PF; Alexander, RS; Hilmer, R; Bostrom, L; Abelman, MM; Lee, SL; Weber, PC; Kettner, CA; Knabb, RM; Wexler, RR (N-acyl-N-alkyl)glycyl borolysine analogs: A new class of potent thrombin inhibitors Bioorg Med Chem Lett6:2913-2918 (1996) Article
Galemmo, RA; Fevig, JM; Carini, DJ; Cacciola, J; Wells, BL; Hillyer, GL; Jr., JB; Rossi, KA; Stouten, PF; Alexander, RS; Hilmer, R; Bostrom, L; Abelman, MM; Lee, SL; Weber, PC; Kettner, CA; Knabb, RM; Wexler, RR (N-acyl-N-alkyl)glycyl borolysine analogs: A new class of potent thrombin inhibitors Bioorg Med Chem Lett6:2913-2918 (1996) Article