 Found 598 hits with Last Name = 'cacciola' and Initial = 'j'
Found 598 hits with Last Name = 'cacciola' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096105
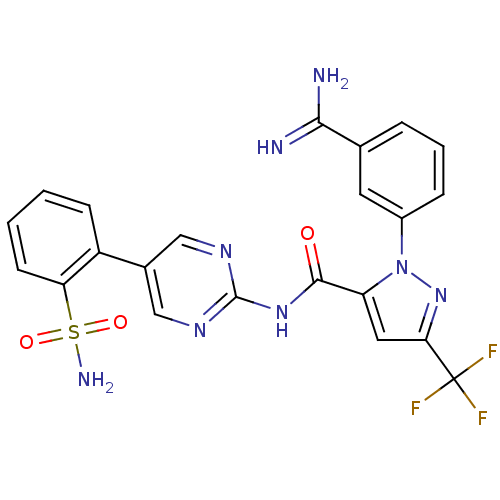
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ncc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C22H17F3N8O3S/c23-22(24,25)18-9-16(33(32-18)14-5-3-4-12(8-14)19(26)27)20(34)31-21-29-10-13(11-30-21)15-6-1-2-7-17(15)37(28,35)36/h1-11H,(H3,26,27)(H2,28,35,36)(H,29,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096099
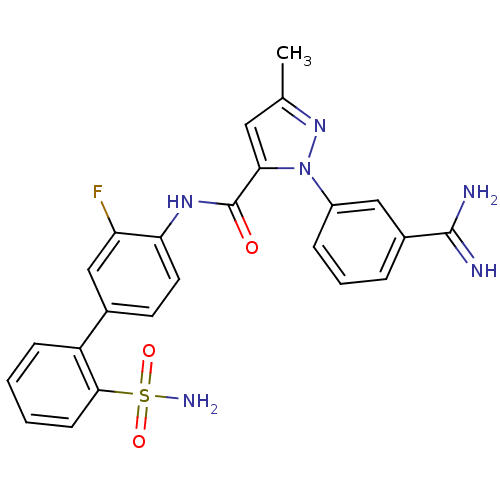
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21FN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096101
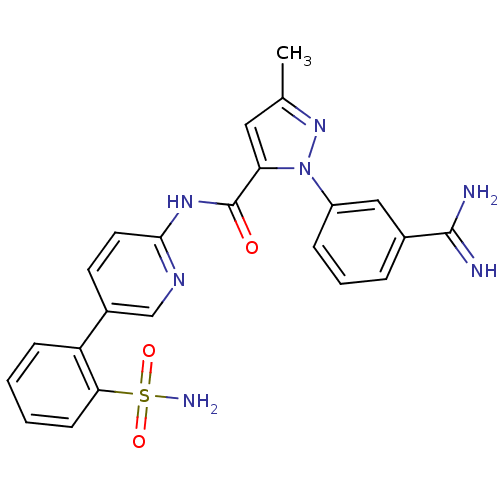
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H21N7O3S/c1-14-11-19(30(29-14)17-6-4-5-15(12-17)22(24)25)23(31)28-21-10-9-16(13-27-21)18-7-2-3-8-20(18)34(26,32)33/h2-13H,1H3,(H3,24,25)(H2,26,32,33)(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096110
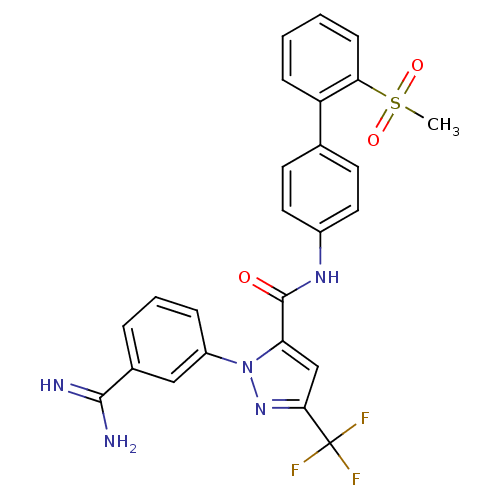
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(c2)C(N)=N)C(F)(F)F)cc1 Show InChI InChI=1S/C25H20F3N5O3S/c1-37(35,36)21-8-3-2-7-19(21)15-9-11-17(12-10-15)31-24(34)20-14-22(25(26,27)28)32-33(20)18-6-4-5-16(13-18)23(29)30/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096091
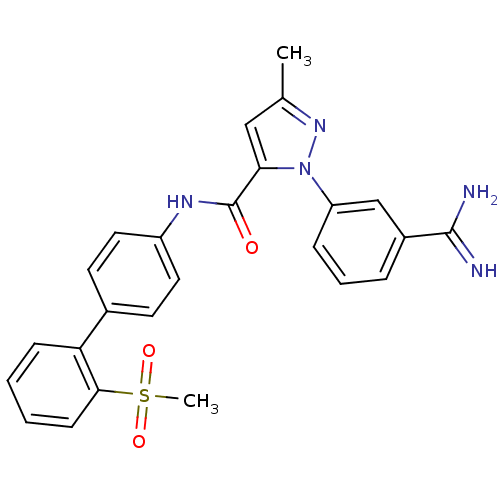
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H23N5O3S/c1-16-14-22(30(29-16)20-7-5-6-18(15-20)24(26)27)25(31)28-19-12-10-17(11-13-19)21-8-3-4-9-23(21)34(2,32)33/h3-15H,1-2H3,(H3,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096085
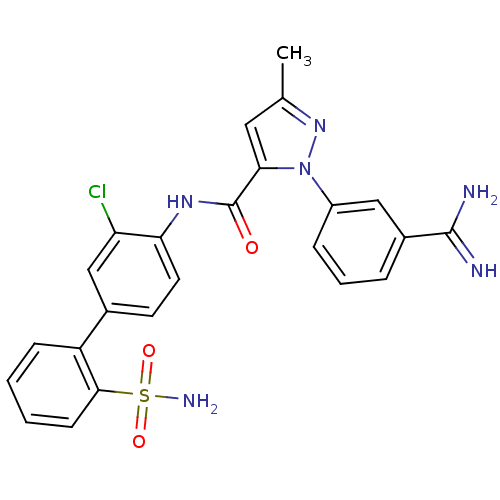
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Cl)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21ClN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096108
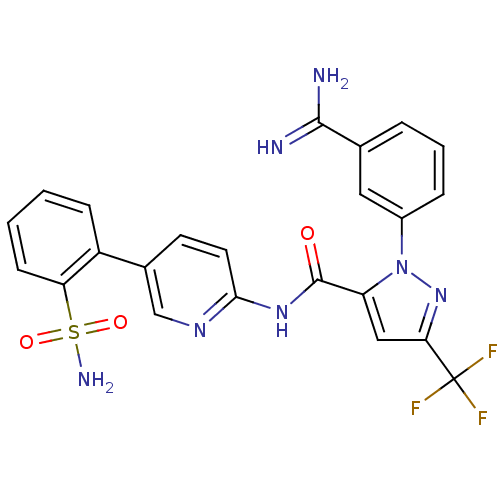
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C23H18F3N7O3S/c24-23(25,26)19-11-17(33(32-19)15-5-3-4-13(10-15)21(27)28)22(34)31-20-9-8-14(12-30-20)16-6-1-2-7-18(16)37(29,35)36/h1-12H,(H3,27,28)(H2,29,35,36)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096098
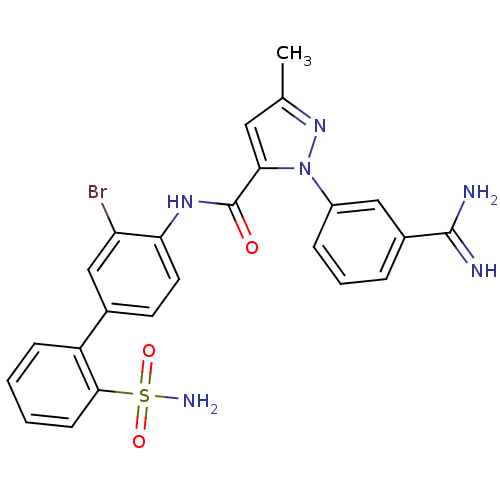
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Br)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21BrN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096111
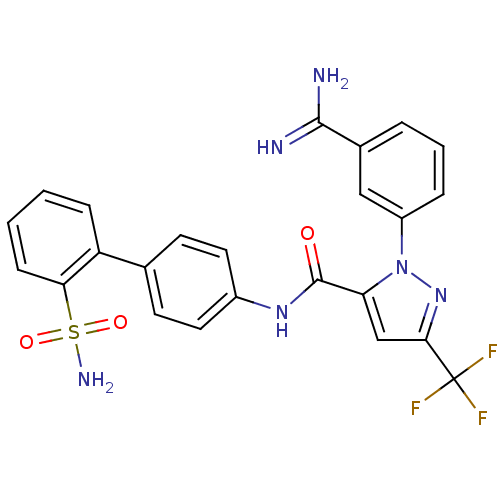
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3N6O3S/c25-24(26,27)21-13-19(33(32-21)17-5-3-4-15(12-17)22(28)29)23(34)31-16-10-8-14(9-11-16)18-6-1-2-7-20(18)37(30,35)36/h1-13H,(H3,28,29)(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096096
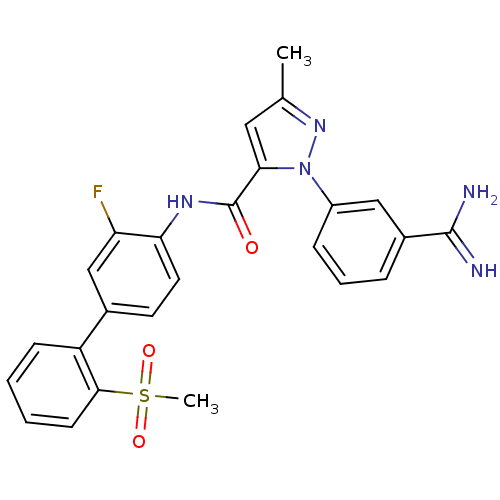
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H22FN5O3S/c1-15-12-22(31(30-15)18-7-5-6-17(13-18)24(27)28)25(32)29-21-11-10-16(14-20(21)26)19-8-3-4-9-23(19)35(2,33)34/h3-14H,1-2H3,(H3,27,28)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro activity against rabbit FXa. |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288632
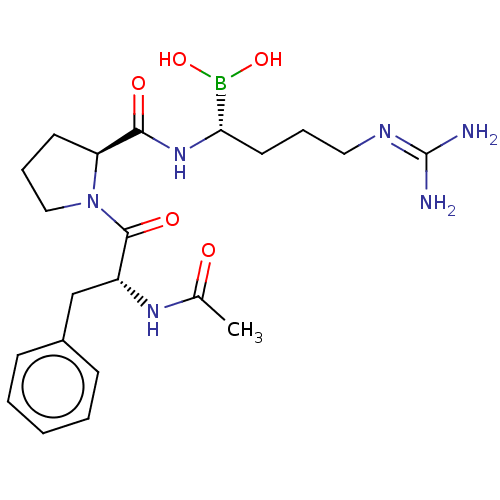
(Boropeptide | CHEMBL607008)Show SMILES Cl.[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#5](-[#8])-[#8] |r| Show InChI InChI=1S/C21H33BN6O5.ClH/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24;/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25);1H/t16-,17+,18+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was tested. |
Bioorg Med Chem Lett 8: 301-6 (1999)
BindingDB Entry DOI: 10.7270/Q2542MRM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096094
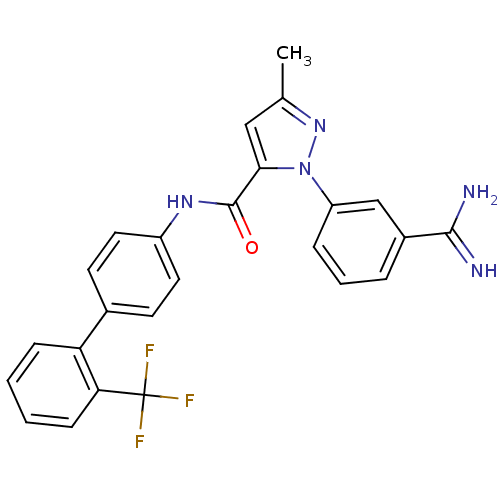
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2C(F)(F)F)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H20F3N5O/c1-15-13-22(33(32-15)19-6-4-5-17(14-19)23(29)30)24(34)31-18-11-9-16(10-12-18)20-7-2-3-8-21(20)25(26,27)28/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288632

(Boropeptide | CHEMBL607008)Show SMILES Cl.[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#5](-[#8])-[#8] |r| Show InChI InChI=1S/C21H33BN6O5.ClH/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24;/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25);1H/t16-,17+,18+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096112
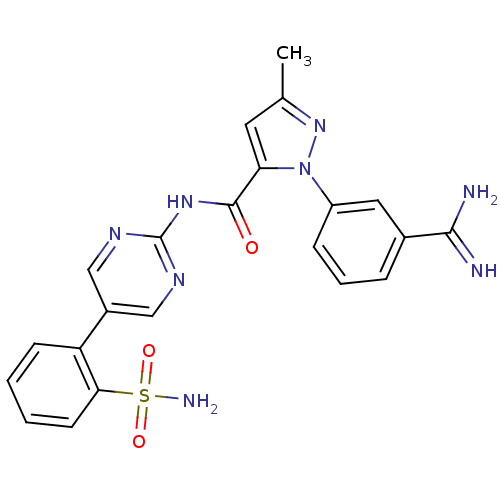
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ncc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C22H20N8O3S/c1-13-9-18(30(29-13)16-6-4-5-14(10-16)20(23)24)21(31)28-22-26-11-15(12-27-22)17-7-2-3-8-19(17)34(25,32)33/h2-12H,1H3,(H3,23,24)(H2,25,32,33)(H,26,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
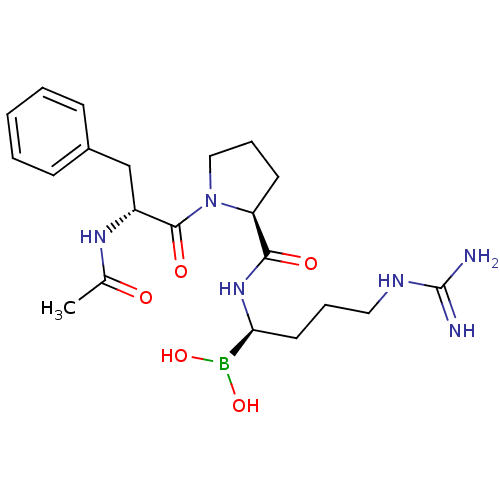
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096090
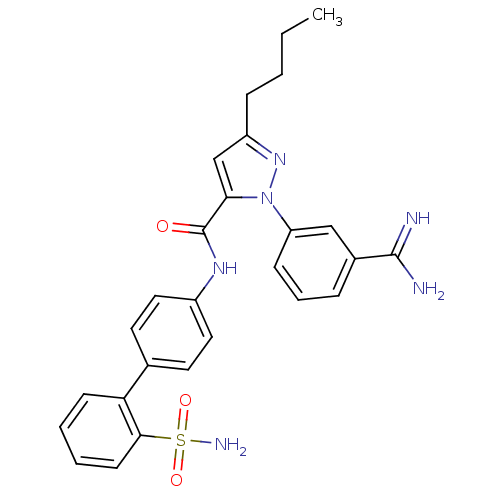
(5-Butyl-2-(3-carbamimidoyl-phenyl)-2H-pyrazole-3-c...)Show SMILES CCCCc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H28N6O3S/c1-2-3-8-21-17-24(33(32-21)22-9-6-7-19(16-22)26(28)29)27(34)31-20-14-12-18(13-15-20)23-10-4-5-11-25(23)37(30,35)36/h4-7,9-17H,2-3,8H2,1H3,(H3,28,29)(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069926

(Boropeptide analogue | CHEMBL105137)Show SMILES CC(C)(CN(CC(=O)NC(CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H38BN5O4/c1-26(2,21-12-7-4-8-13-21)19-32(24(34)16-15-20-10-5-3-6-11-20)18-23(33)31-22(27(35)36)14-9-17-30-25(28)29/h3-8,10-13,22,35-36H,9,14-19H2,1-2H3,(H,31,33)(H4,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was tested. |
Bioorg Med Chem Lett 8: 301-6 (1999)
BindingDB Entry DOI: 10.7270/Q2542MRM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069928

(Boropeptide analogue | CHEMBL102385)Show SMILES NC(=N)NCCCC(NC(=O)CN(CC1(CC1)c1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C26H36BN5O4/c28-25(29)30-17-7-12-22(27(35)36)31-23(33)18-32(24(34)14-13-20-8-3-1-4-9-20)19-26(15-16-26)21-10-5-2-6-11-21/h1-6,8-11,22,35-36H,7,12-19H2,(H,31,33)(H4,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was tested. |
Bioorg Med Chem Lett 8: 301-6 (1999)
BindingDB Entry DOI: 10.7270/Q2542MRM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288621
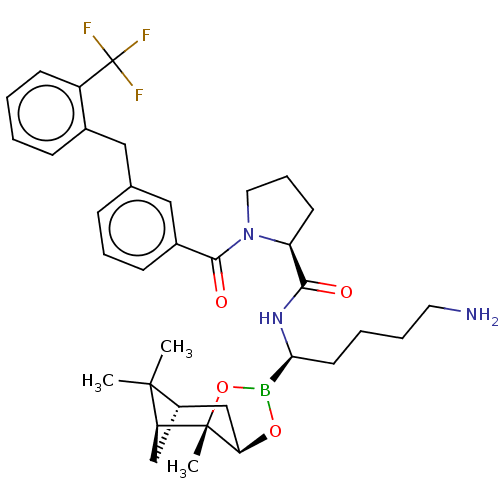
((S)-1-[3-(2-Trifluoromethyl-benzyl)-benzoyl]-pyrro...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccccc2C(F)(F)F)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C35H45BF3N3O4.ClH/c1-33(2)25-20-28(33)34(3)29(21-25)45-36(46-34)30(15-6-7-16-40)41-31(43)27-14-9-17-42(27)32(44)24-12-8-10-22(19-24)18-23-11-4-5-13-26(23)35(37,38)39;/h4-5,8,10-13,19,25,27-30H,6-7,9,14-18,20-21,40H2,1-3H3,(H,41,43);1H/t25-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288628
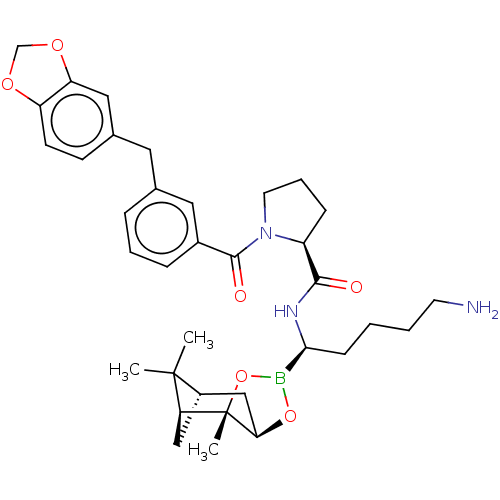
((S)-1-(3-Benzo[1,3]dioxol-5-ylmethyl-benzoyl)-pyrr...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccc3OCOc3c2)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C35H46BN3O6.ClH/c1-34(2)25-19-29(34)35(3)30(20-25)44-36(45-35)31(11-4-5-14-37)38-32(40)26-10-7-15-39(26)33(41)24-9-6-8-22(17-24)16-23-12-13-27-28(18-23)43-21-42-27;/h6,8-9,12-13,17-18,25-26,29-31H,4-5,7,10-11,14-16,19-21,37H2,1-3H3,(H,38,40);1H/t25-,26-,29-,30+,31-,35-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50000020
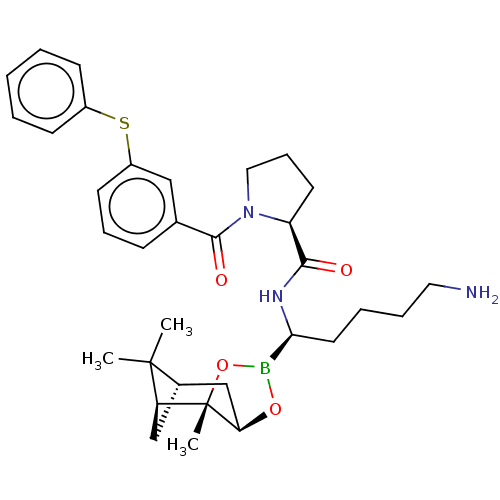
(CHEMBL2448361)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Sc2ccccc2)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C33H44BN3O4S.ClH/c1-32(2)23-20-27(32)33(3)28(21-23)40-34(41-33)29(16-7-8-17-35)36-30(38)26-15-10-18-37(26)31(39)22-11-9-14-25(19-22)42-24-12-5-4-6-13-24;/h4-6,9,11-14,19,23,26-29H,7-8,10,15-18,20-21,35H2,1-3H3,(H,36,38);1H/t23-,26-,27-,28+,29-,33-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12870
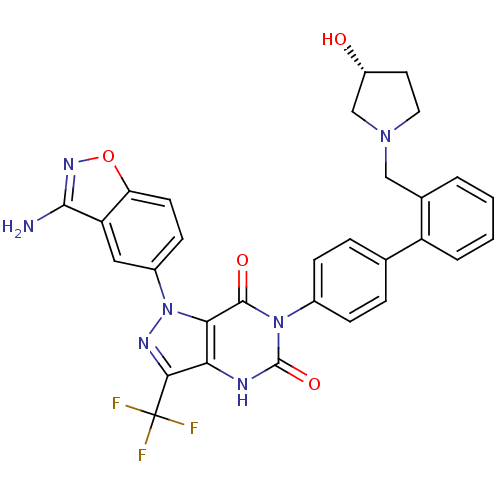
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2[nH]c(=O)n(-c3ccc(cc3)-c3ccccc3CN3CC[C@@H](O)C3)c(=O)c12)C(F)(F)F |r| Show InChI InChI=1S/C30H24F3N7O4/c31-30(32,33)26-24-25(40(36-26)19-9-10-23-22(13-19)27(34)37-44-23)28(42)39(29(43)35-24)18-7-5-16(6-8-18)21-4-2-1-3-17(21)14-38-12-11-20(41)15-38/h1-10,13,20,41H,11-12,14-15H2,(H2,34,37)(H,35,43)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087533
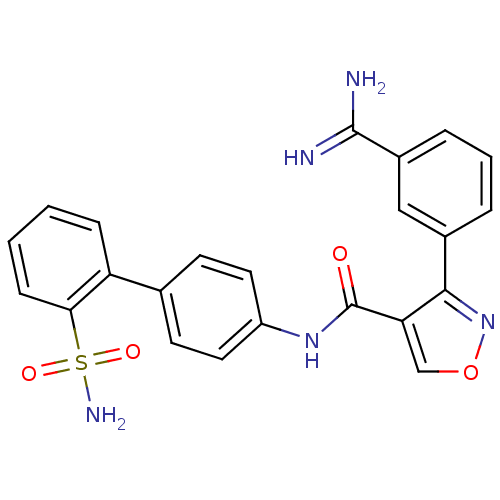
(3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...)Show SMILES NC(=N)c1cccc(c1)-c1nocc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H19N5O4S/c24-22(25)16-5-3-4-15(12-16)21-19(13-32-28-21)23(29)27-17-10-8-14(9-11-17)18-6-1-2-7-20(18)33(26,30)31/h1-13H,(H3,24,25)(H,27,29)(H2,26,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096095
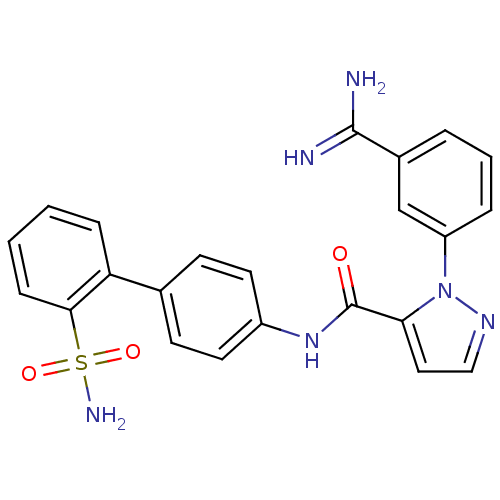
(2-(3-Carbamimidoyl-phenyl)-2H-pyrazole-3-carboxyli...)Show SMILES NC(=N)c1cccc(c1)-n1nccc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H20N6O3S/c24-22(25)16-4-3-5-18(14-16)29-20(12-13-27-29)23(30)28-17-10-8-15(9-11-17)19-6-1-2-7-21(19)33(26,31)32/h1-14H,(H3,24,25)(H,28,30)(H2,26,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288631
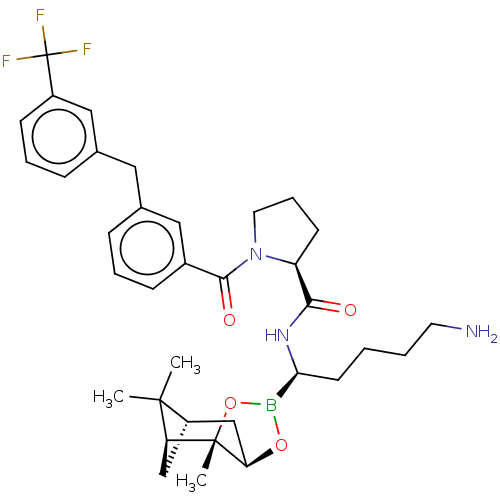
((S)-1-[3-(3-Trifluoromethyl-benzyl)-benzoyl]-pyrro...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2cccc(c2)C(F)(F)F)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C35H45BF3N3O4.ClH/c1-33(2)26-20-28(33)34(3)29(21-26)45-36(46-34)30(14-4-5-15-40)41-31(43)27-13-8-16-42(27)32(44)24-11-6-9-22(18-24)17-23-10-7-12-25(19-23)35(37,38)39;/h6-7,9-12,18-19,26-30H,4-5,8,13-17,20-21,40H2,1-3H3,(H,41,43);1H/t26-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288405
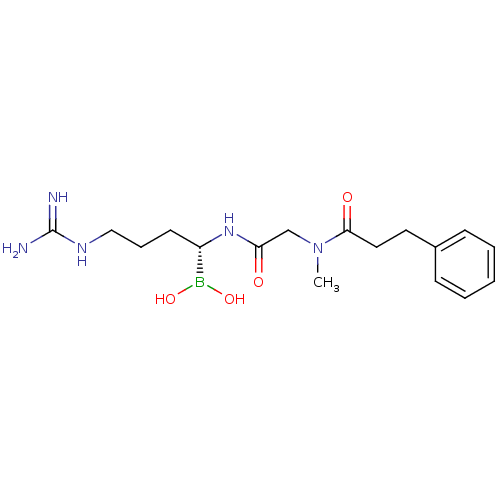
(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12864
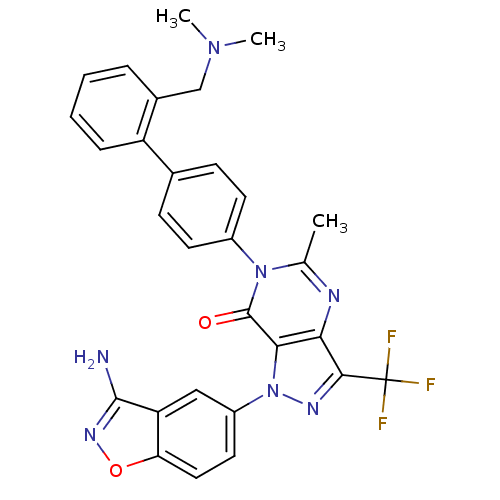
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1c(C)nc2c(nn(-c3ccc4onc(N)c4c3)c2c1=O)C(F)(F)F Show InChI InChI=1S/C29H24F3N7O2/c1-16-34-24-25(39(35-26(24)29(30,31)32)20-12-13-23-22(14-20)27(33)36-41-23)28(40)38(16)19-10-8-17(9-11-19)21-7-5-4-6-18(21)15-37(2)3/h4-14H,15H2,1-3H3,(H2,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12866
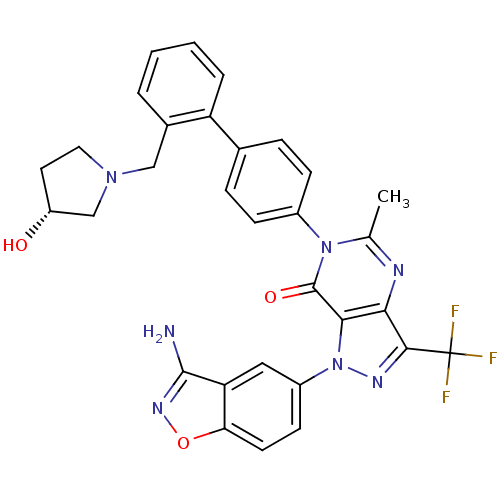
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Cc1nc2c(nn(-c3ccc4onc(N)c4c3)c2c(=O)n1-c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H26F3N7O3/c1-17-36-26-27(41(37-28(26)31(32,33)34)21-10-11-25-24(14-21)29(35)38-44-25)30(43)40(17)20-8-6-18(7-9-20)23-5-3-2-4-19(23)15-39-13-12-22(42)16-39/h2-11,14,22,42H,12-13,15-16H2,1H3,(H2,35,38)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288634
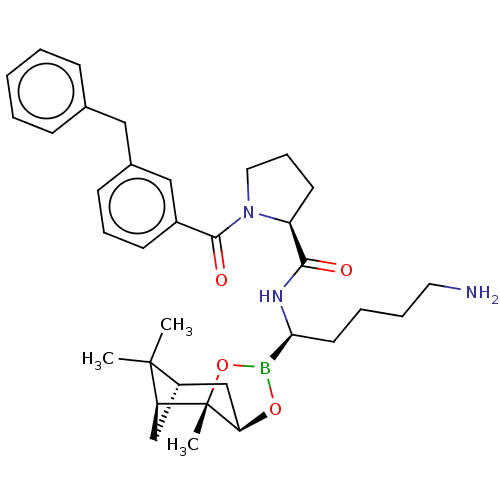
((S)-1-(3-Benzyl-benzoyl)-pyrrolidine-2-carboxylic ...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccccc2)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C34H46BN3O4.ClH/c1-33(2)26-21-28(33)34(3)29(22-26)41-35(42-34)30(16-7-8-17-36)37-31(39)27-15-10-18-38(27)32(40)25-14-9-13-24(20-25)19-23-11-5-4-6-12-23;/h4-6,9,11-14,20,26-30H,7-8,10,15-19,21-22,36H2,1-3H3,(H,37,39);1H/t26-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288623
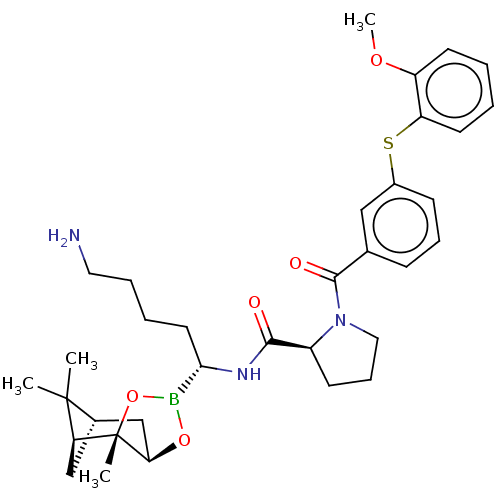
((S)-1-[3-(2-Methoxy-phenylsulfanyl)-benzoyl]-pyrro...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Sc2ccccc2OC)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C34H46BN3O5S.ClH/c1-33(2)23-20-28(33)34(3)29(21-23)42-35(43-34)30(16-7-8-17-36)37-31(39)25-13-10-18-38(25)32(40)22-11-9-12-24(19-22)44-27-15-6-5-14-26(27)41-4;/h5-6,9,11-12,14-15,19,23,25,28-30H,7-8,10,13,16-18,20-21,36H2,1-4H3,(H,37,39);1H/t23-,25-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 3755-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.044
BindingDB Entry DOI: 10.7270/Q2319T41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12868
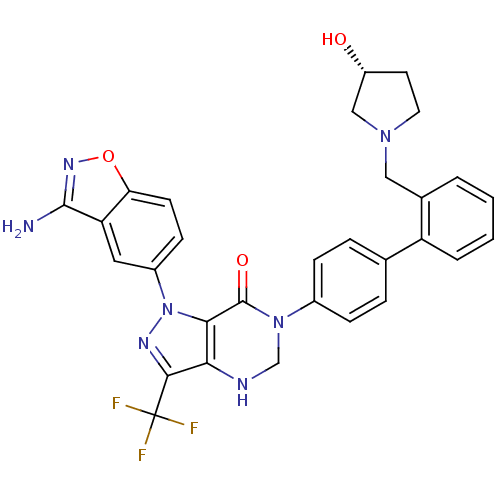
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2NCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C30H26F3N7O3/c31-30(32,33)27-25-26(40(36-27)20-9-10-24-23(13-20)28(34)37-43-24)29(42)39(16-35-25)19-7-5-17(6-8-19)22-4-2-1-3-18(22)14-38-12-11-21(41)15-38/h1-10,13,21,35,41H,11-12,14-16H2,(H2,34,37)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12871
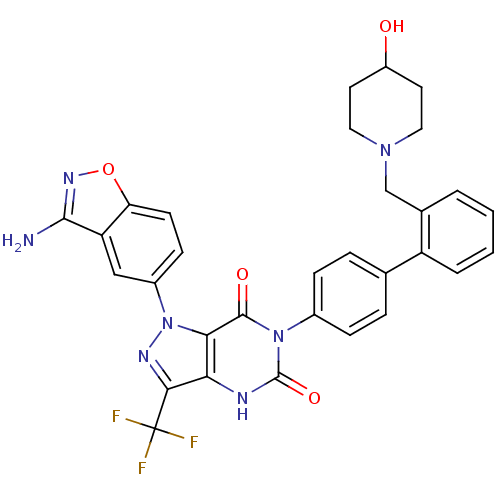
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydrox...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2[nH]c(=O)n(-c3ccc(cc3)-c3ccccc3CN3CCC(O)CC3)c(=O)c12)C(F)(F)F Show InChI InChI=1S/C31H26F3N7O4/c32-31(33,34)27-25-26(41(37-27)20-9-10-24-23(15-20)28(35)38-45-24)29(43)40(30(44)36-25)19-7-5-17(6-8-19)22-4-2-1-3-18(22)16-39-13-11-21(42)12-14-39/h1-10,15,21,42H,11-14,16H2,(H2,35,38)(H,36,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288627
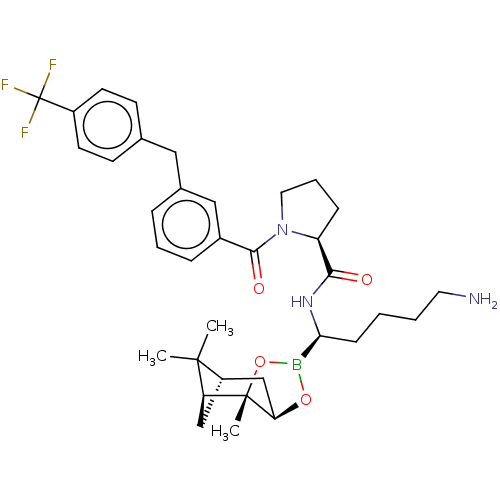
((S)-1-[3-(4-Trifluoromethyl-benzyl)-benzoyl]-pyrro...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccc(cc2)C(F)(F)F)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C35H45BF3N3O4.ClH/c1-33(2)26-20-28(33)34(3)29(21-26)45-36(46-34)30(11-4-5-16-40)41-31(43)27-10-7-17-42(27)32(44)24-9-6-8-23(19-24)18-22-12-14-25(15-13-22)35(37,38)39;/h6,8-9,12-15,19,26-30H,4-5,7,10-11,16-18,20-21,40H2,1-3H3,(H,41,43);1H/t26-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288629
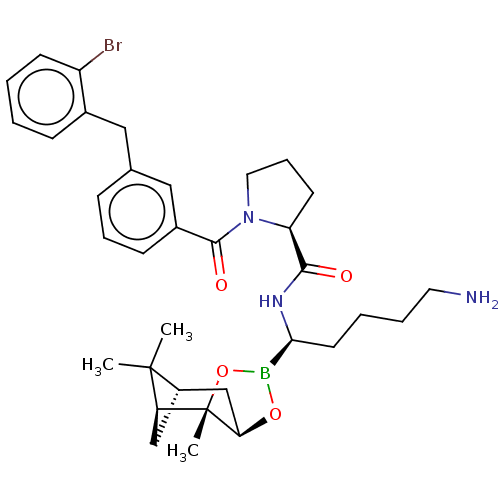
((S)-1-[3-(2-Bromo-benzyl)-benzoyl]-pyrrolidine-2-c...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccccc2Br)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C34H45BBrN3O4.ClH/c1-33(2)25-20-28(33)34(3)29(21-25)42-35(43-34)30(15-6-7-16-37)38-31(40)27-14-9-17-39(27)32(41)24-12-8-10-22(19-24)18-23-11-4-5-13-26(23)36;/h4-5,8,10-13,19,25,27-30H,6-7,9,14-18,20-21,37H2,1-3H3,(H,38,40);1H/t25-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288414
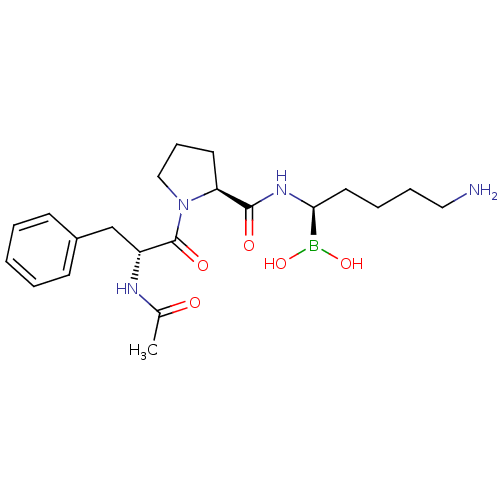
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)B(O)O Show InChI InChI=1S/C21H33BN4O5/c1-15(27)24-17(14-16-8-3-2-4-9-16)21(29)26-13-7-10-18(26)20(28)25-19(22(30)31)11-5-6-12-23/h2-4,8-9,17-19,30-31H,5-7,10-14,23H2,1H3,(H,24,27)(H,25,28)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50288620
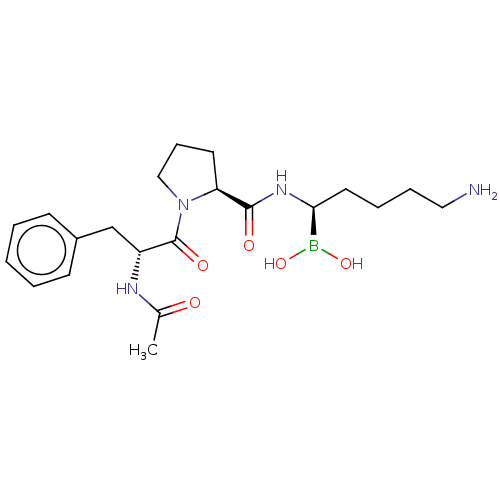
(Boropeptide | CHEMBL3038261)Show SMILES Cl.CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)B(O)O Show InChI InChI=1S/C21H33BN4O5.ClH/c1-15(27)24-17(14-16-8-3-2-4-9-16)21(29)26-13-7-10-18(26)20(28)25-19(22(30)31)11-5-6-12-23;/h2-4,8-9,17-19,30-31H,5-7,10-14,23H2,1H3,(H,24,27)(H,25,28);1H/t17-,18+,19+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288637
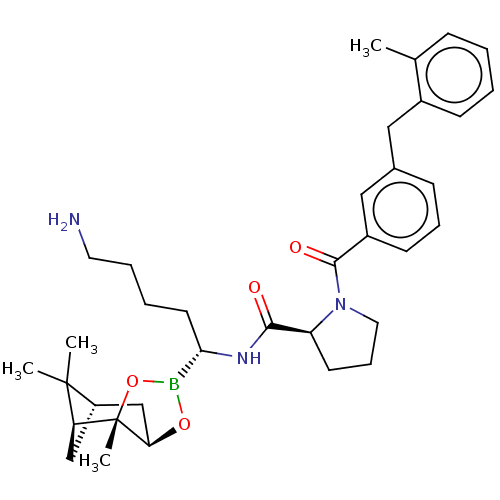
((S)-1-[3-(2-Methyl-benzyl)-benzoyl]-pyrrolidine-2-...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1cccc(Cc2ccccc2C)c1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C35H48BN3O4.ClH/c1-23-11-5-6-13-25(23)19-24-12-9-14-26(20-24)33(41)39-18-10-15-28(39)32(40)38-31(16-7-8-17-37)36-42-30-22-27-21-29(34(27,2)3)35(30,4)43-36;/h5-6,9,11-14,20,27-31H,7-8,10,15-19,21-22,37H2,1-4H3,(H,38,40);1H/t27-,28-,29-,30+,31-,35-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12687
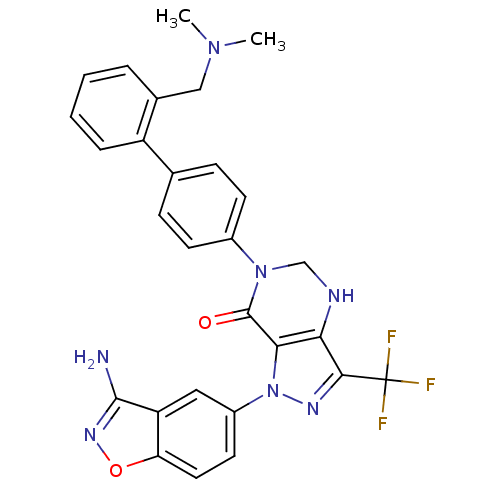
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CNc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O2/c1-36(2)14-17-5-3-4-6-20(17)16-7-9-18(10-8-16)37-15-33-23-24(27(37)39)38(34-25(23)28(29,30)31)19-11-12-22-21(13-19)26(32)35-40-22/h3-13,33H,14-15H2,1-2H3,(H2,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288630
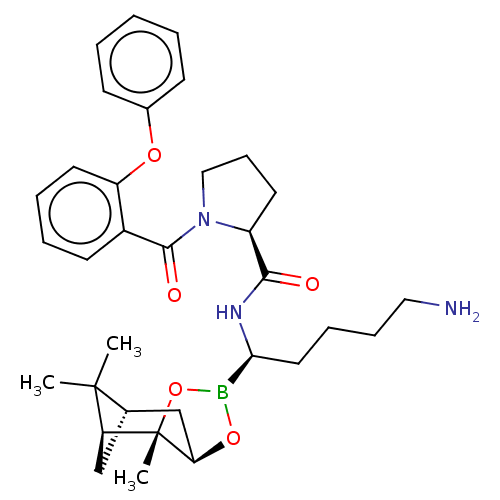
((S)-1-(2-Phenoxy-benzoyl)-pyrrolidine-2-carboxylic...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1Oc1ccccc1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C33H44BN3O5.ClH/c1-32(2)22-20-27(32)33(3)28(21-22)41-34(42-33)29(17-9-10-18-35)36-30(38)25-15-11-19-37(25)31(39)24-14-7-8-16-26(24)40-23-12-5-4-6-13-23;/h4-8,12-14,16,22,25,27-29H,9-11,15,17-21,35H2,1-3H3,(H,36,38);1H/t22-,25-,27-,28+,29-,33-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12860
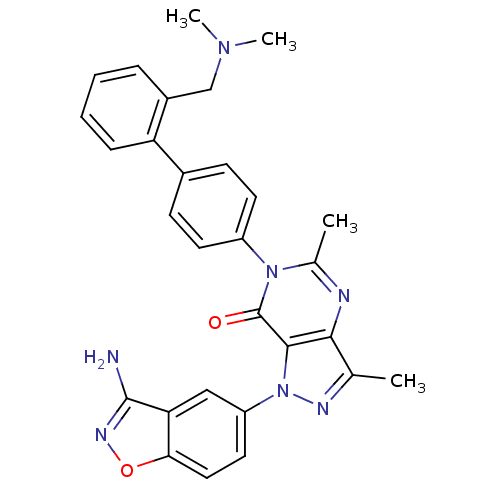
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1c(C)nc2c(C)nn(-c3ccc4onc(N)c4c3)c2c1=O Show InChI InChI=1S/C29H27N7O2/c1-17-26-27(36(32-17)22-13-14-25-24(15-22)28(30)33-38-25)29(37)35(18(2)31-26)21-11-9-19(10-12-21)23-8-6-5-7-20(23)16-34(3)4/h5-15H,16H2,1-4H3,(H2,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069933
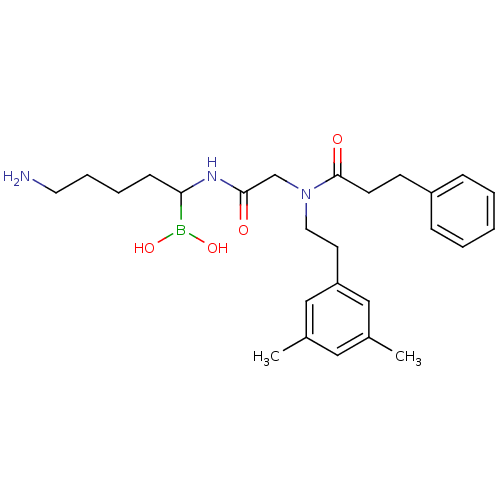
(Boropeptide analogue | CHEMBL102916)Show SMILES Cc1cc(C)cc(CCN(CC(=O)NC(CCCCN)B(O)O)C(=O)CCc2ccccc2)c1 Show InChI InChI=1S/C26H38BN3O4/c1-20-16-21(2)18-23(17-20)13-15-30(26(32)12-11-22-8-4-3-5-9-22)19-25(31)29-24(27(33)34)10-6-7-14-28/h3-5,8-9,16-18,24,33-34H,6-7,10-15,19,28H2,1-2H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was tested. |
Bioorg Med Chem Lett 8: 301-6 (1999)
BindingDB Entry DOI: 10.7270/Q2542MRM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288636
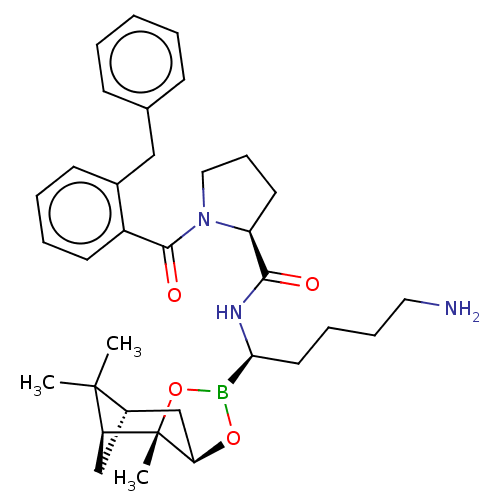
((S)-1-(2-Benzyl-benzoyl)-pyrrolidine-2-carboxylic ...)Show SMILES Cl.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1Cc1ccccc1 |TLB:10:9:6:3,THB:11:9:6:3,13:14:6:3| Show InChI InChI=1S/C34H46BN3O4.ClH/c1-33(2)25-21-28(33)34(3)29(22-25)41-35(42-34)30(17-9-10-18-36)37-31(39)27-16-11-19-38(27)32(40)26-15-8-7-14-24(26)20-23-12-5-4-6-13-23;/h4-8,12-15,25,27-30H,9-11,16-22,36H2,1-3H3,(H,37,39);1H/t25-,27-,28-,29+,30-,34-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin. |
Bioorg Med Chem Lett 6: 301-306 (1996)
Article DOI: 10.1016/0960-894X(96)00016-9
BindingDB Entry DOI: 10.7270/Q2959HJX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096102
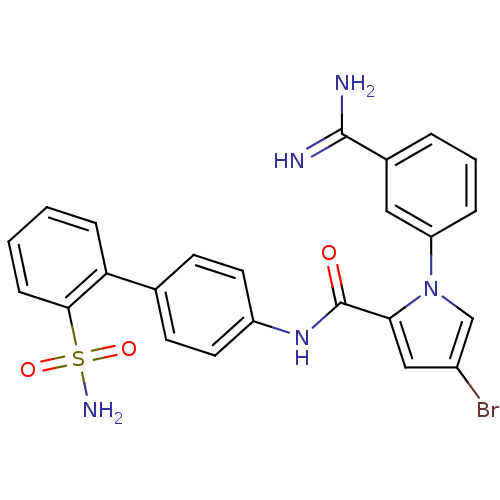
(4-Bromo-1-(3-carbamimidoyl-phenyl)-1H-pyrrole-2-ca...)Show SMILES NC(=N)c1cccc(c1)-n1cc(Br)cc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C24H20BrN5O3S/c25-17-13-21(30(14-17)19-5-3-4-16(12-19)23(26)27)24(31)29-18-10-8-15(9-11-18)20-6-1-2-7-22(20)34(28,32)33/h1-14H,(H3,26,27)(H,29,31)(H2,28,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496270

(CHEMBL3127679)Show SMILES CC(C)N1CCN(CC1)C(=O)C1CC2(C1)CCN(CC2)C1CCOCC1 Show InChI InChI=1S/C21H37N3O2/c1-17(2)22-9-11-24(12-10-22)20(25)18-15-21(16-18)5-7-23(8-6-21)19-3-13-26-14-4-19/h17-19H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069935

(Boropeptide analogue | CHEMBL320213)Show SMILES Cc1cc(C)cc(c1)C(C)(C)CN(CC(=O)NC(CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C28H42BN5O4/c1-20-15-21(2)17-23(16-20)28(3,4)19-34(26(36)13-12-22-9-6-5-7-10-22)18-25(35)33-24(29(37)38)11-8-14-32-27(30)31/h5-7,9-10,15-17,24,37-38H,8,11-14,18-19H2,1-4H3,(H,33,35)(H4,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was tested. |
Bioorg Med Chem Lett 8: 301-6 (1999)
BindingDB Entry DOI: 10.7270/Q2542MRM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12657
(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro activity against rabbit factor Xa |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data