 Found 109 hits with Last Name = 'lee' and Initial = 'sl'
Found 109 hits with Last Name = 'lee' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
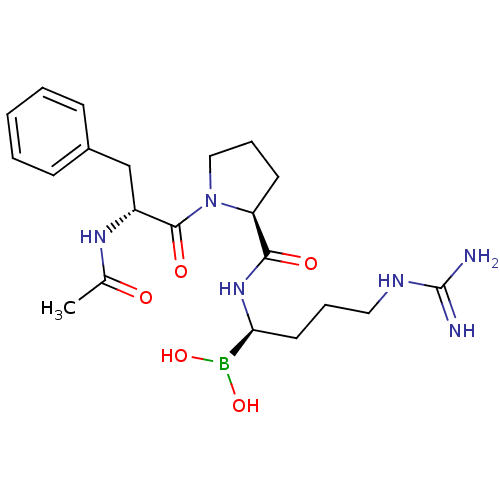
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50421510
(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Sunflower beta-trypsin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288405
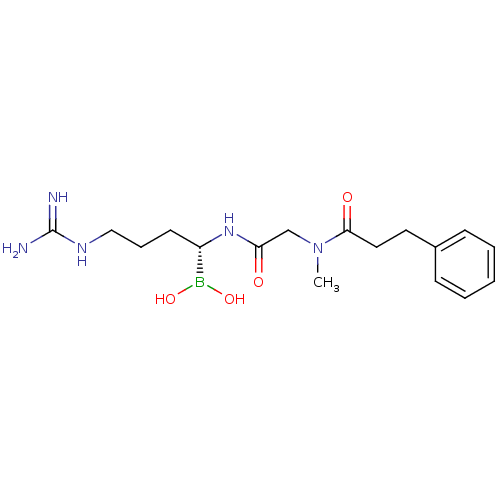
(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288414
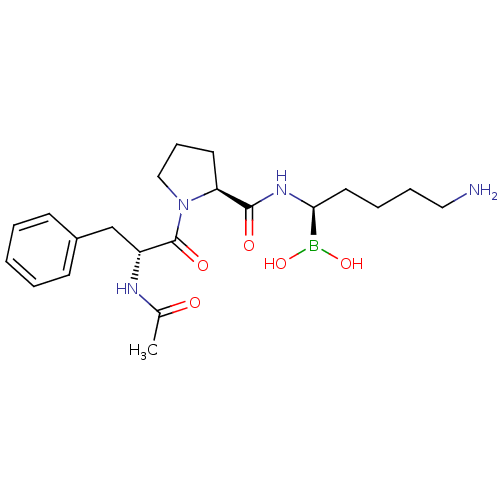
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)B(O)O Show InChI InChI=1S/C21H33BN4O5/c1-15(27)24-17(14-16-8-3-2-4-9-16)21(29)26-13-7-10-18(26)20(28)25-19(22(30)31)11-5-6-12-23/h2-4,8-9,17-19,30-31H,5-7,10-14,23H2,1H3,(H,24,27)(H,25,28)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50288409
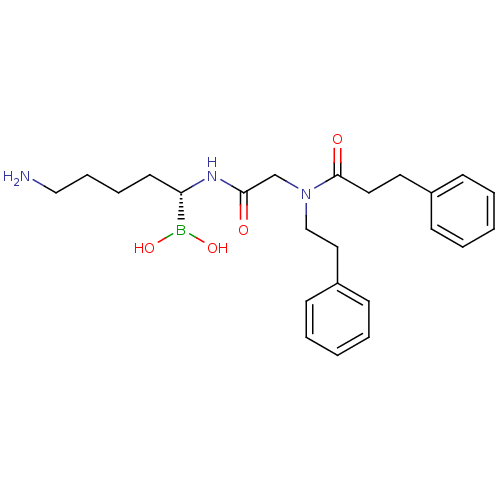
(CHEMBL99309 | N-[(5-Amino-1-dihydroxyboranyl-penty...)Show SMILES NCCCC[C@H](NC(=O)CN(CCc1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C24H34BN3O4/c26-17-8-7-13-22(25(31)32)27-23(29)19-28(18-16-21-11-5-2-6-12-21)24(30)15-14-20-9-3-1-4-10-20/h1-6,9-12,22,31-32H,7-8,13-19,26H2,(H,27,29)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288405

(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288407
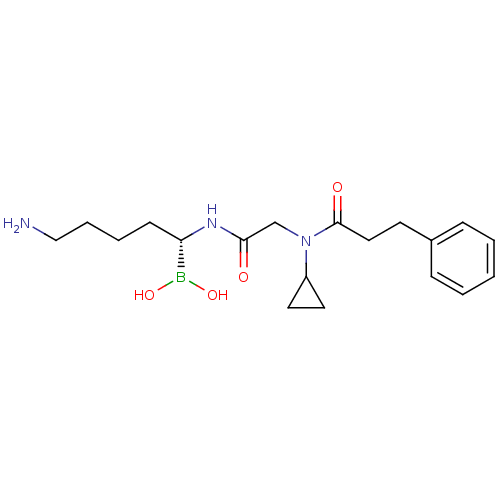
(CHEMBL317137 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-8-17(20(26)27)22-18(24)14-23(16-10-11-16)19(25)12-9-15-6-2-1-3-7-15/h1-3,6-7,16-17,26-27H,4-5,8-14,21H2,(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288408
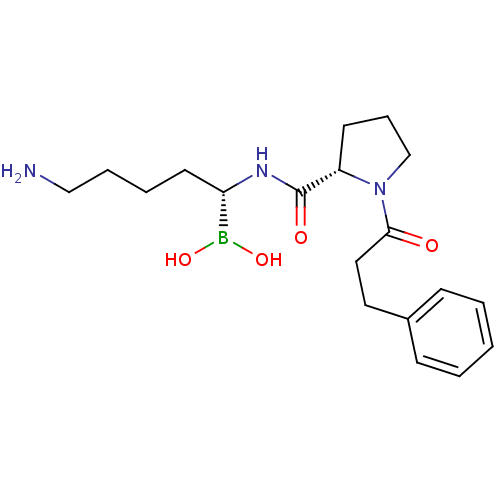
(1-(3-Phenyl-propionyl)-pyrrolidine-2-carboxylic ac...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-10-17(20(26)27)22-19(25)16-9-6-14-23(16)18(24)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-17,26-27H,4-6,9-14,21H2,(H,22,25)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Matriptase from human breast cancer cells |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Trypsin-3
(Homo sapiens (Human)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of bovine beta trypsin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288407

(CHEMBL317137 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-8-17(20(26)27)22-18(24)14-23(16-10-11-16)19(25)12-9-15-6-2-1-3-7-15/h1-3,6-7,16-17,26-27H,4-5,8-14,21H2,(H,22,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288413
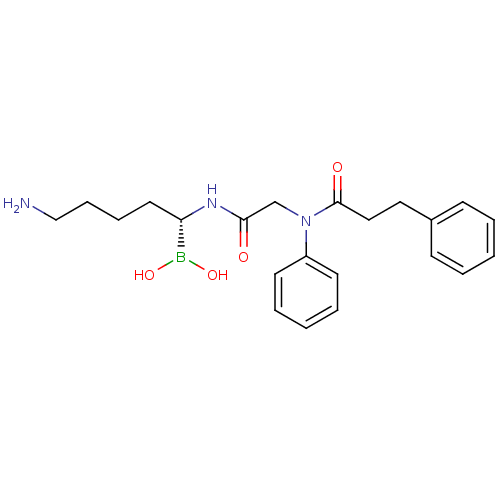
(CHEMBL101759 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C(=O)CCc1ccccc1)c1ccccc1)B(O)O Show InChI InChI=1S/C22H30BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1-6,9-12,20,29-30H,7-8,13-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288403
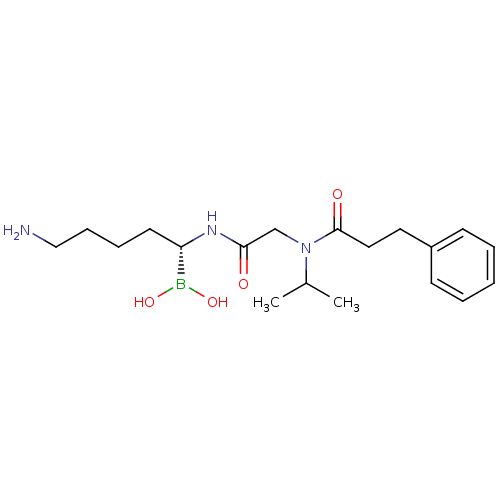
(CHEMBL330206 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CC(C)N(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C19H32BN3O4/c1-15(2)23(19(25)12-11-16-8-4-3-5-9-16)14-18(24)22-17(20(26)27)10-6-7-13-21/h3-5,8-9,15,17,26-27H,6-7,10-14,21H2,1-2H3,(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069174
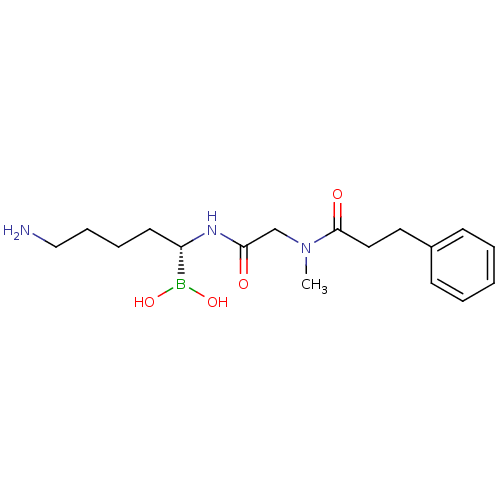
(Boronate Ester analogue | CHEMBL317682 | N-[(5-Ami...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN3O4/c1-21(17(23)11-10-14-7-3-2-4-8-14)13-16(22)20-15(18(24)25)9-5-6-12-19/h2-4,7-8,15,24-25H,5-6,9-13,19H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288402
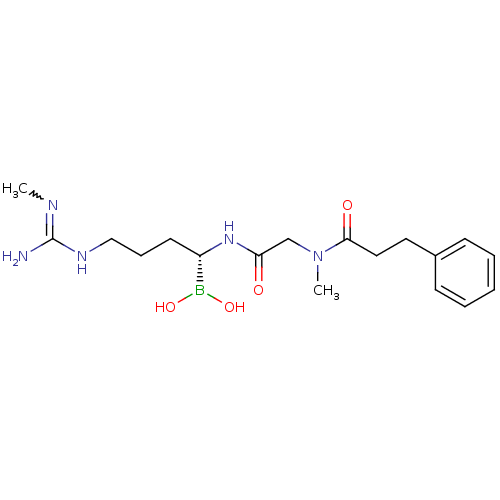
(Borolysine analogue | CHEMBL431814)Show SMILES CN=C(N)NCCC[C@H](NC(=O)CN(C)C(=O)CCc1ccccc1)B(O)O |w:1.0| Show InChI InChI=1S/C18H30BN5O4/c1-21-18(20)22-12-6-9-15(19(27)28)23-16(25)13-24(2)17(26)11-10-14-7-4-3-5-8-14/h3-5,7-8,15,27-28H,6,9-13H2,1-2H3,(H,23,25)(H3,20,21,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288409

(CHEMBL99309 | N-[(5-Amino-1-dihydroxyboranyl-penty...)Show SMILES NCCCC[C@H](NC(=O)CN(CCc1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C24H34BN3O4/c26-17-8-7-13-22(25(31)32)27-23(29)19-28(18-16-21-11-5-2-6-12-21)24(30)15-14-20-9-3-1-4-10-20/h1-6,9-12,22,31-32H,7-8,13-19,26H2,(H,27,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50069174

(Boronate Ester analogue | CHEMBL317682 | N-[(5-Ami...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN3O4/c1-21(17(23)11-10-14-7-3-2-4-8-14)13-16(22)20-15(18(24)25)9-5-6-12-19/h2-4,7-8,15,24-25H,5-6,9-13,19H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288403

(CHEMBL330206 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CC(C)N(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C19H32BN3O4/c1-15(2)23(19(25)12-11-16-8-4-3-5-9-16)14-18(24)22-17(20(26)27)10-6-7-13-21/h3-5,8-9,15,17,26-27H,6-7,10-14,21H2,1-2H3,(H,22,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288404
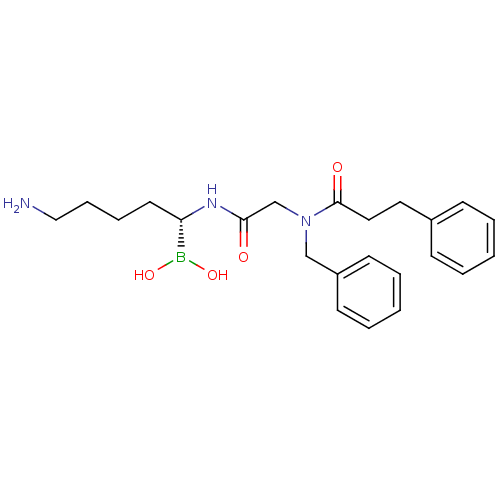
(CHEMBL101707 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(Cc1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C23H32BN3O4/c25-16-8-7-13-21(24(30)31)26-22(28)18-27(17-20-11-5-2-6-12-20)23(29)15-14-19-9-3-1-4-10-19/h1-6,9-12,21,30-31H,7-8,13-18,25H2,(H,26,28)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288400
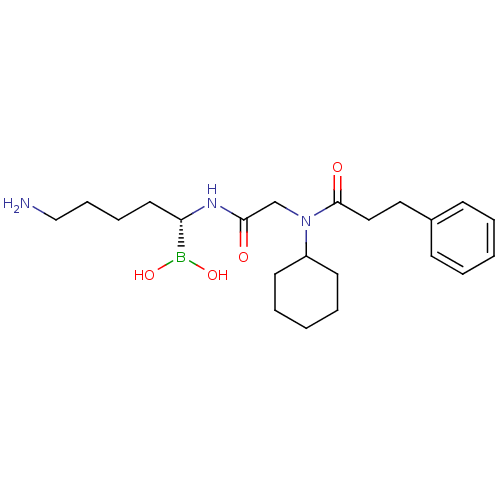
(CHEMBL419892 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CCCCC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C22H36BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1,3-4,9-10,19-20,29-30H,2,5-8,11-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288412
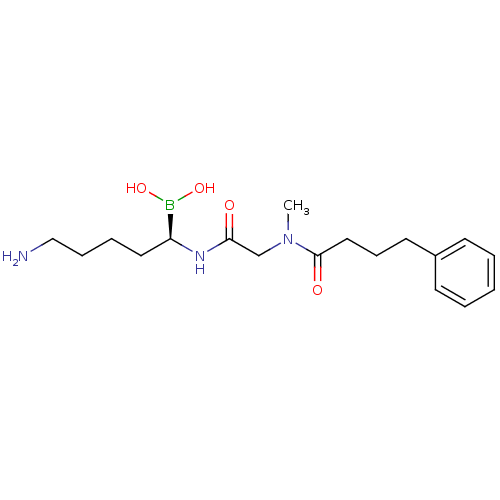
(CHEMBL330149 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCCc1ccccc1 Show InChI InChI=1S/C18H30BN3O4/c1-22(18(24)12-7-10-15-8-3-2-4-9-15)14-17(23)21-16(19(25)26)11-5-6-13-20/h2-4,8-9,16,25-26H,5-7,10-14,20H2,1H3,(H,21,23)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of tissue plasminogen activator |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288400

(CHEMBL419892 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CCCCC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C22H36BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1,3-4,9-10,19-20,29-30H,2,5-8,11-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288410
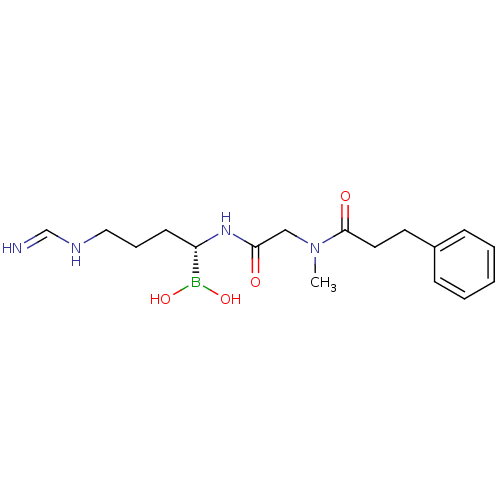
(CHEMBL95993 | N-[(1-Dihydroxyboranyl-4-formimidoyl...)Show SMILES CN(CC(=O)N[C@@H](CCCNC=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H27BN4O4/c1-22(17(24)10-9-14-6-3-2-4-7-14)12-16(23)21-15(18(25)26)8-5-11-20-13-19/h2-4,6-7,13,15,25-26H,5,8-12H2,1H3,(H2,19,20)(H,21,23)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288411
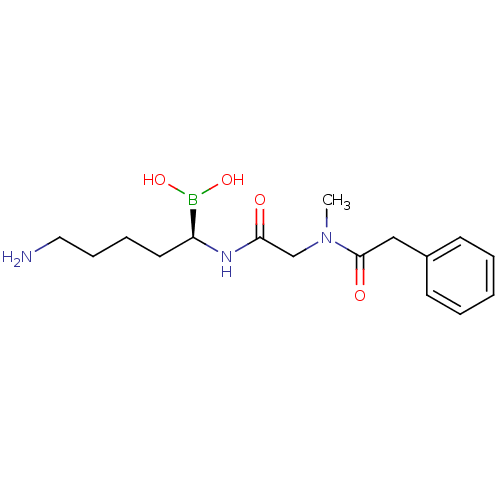
(CHEMBL100187 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C16H26BN3O4/c1-20(16(22)11-13-7-3-2-4-8-13)12-15(21)19-14(17(23)24)9-5-6-10-18/h2-4,7-8,14,23-24H,5-6,9-12,18H2,1H3,(H,19,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288402

(Borolysine analogue | CHEMBL431814)Show SMILES CN=C(N)NCCC[C@H](NC(=O)CN(C)C(=O)CCc1ccccc1)B(O)O |w:1.0| Show InChI InChI=1S/C18H30BN5O4/c1-21-18(20)22-12-6-9-15(19(27)28)23-16(25)13-24(2)17(26)11-10-14-7-4-3-5-8-14/h3-5,7-8,15,27-28H,6,9-13H2,1-2H3,(H,23,25)(H3,20,21,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288410

(CHEMBL95993 | N-[(1-Dihydroxyboranyl-4-formimidoyl...)Show SMILES CN(CC(=O)N[C@@H](CCCNC=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H27BN4O4/c1-22(17(24)10-9-14-6-3-2-4-7-14)12-16(23)21-15(18(25)26)8-5-11-20-13-19/h2-4,6-7,13,15,25-26H,5,8-12H2,1H3,(H2,19,20)(H,21,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of trypsin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50288405

(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of tissue plasminogen activator |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288405

(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288408

(1-(3-Phenyl-propionyl)-pyrrolidine-2-carboxylic ac...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-10-17(20(26)27)22-19(25)16-9-6-14-23(16)18(24)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-17,26-27H,4-6,9-14,21H2,(H,22,25)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
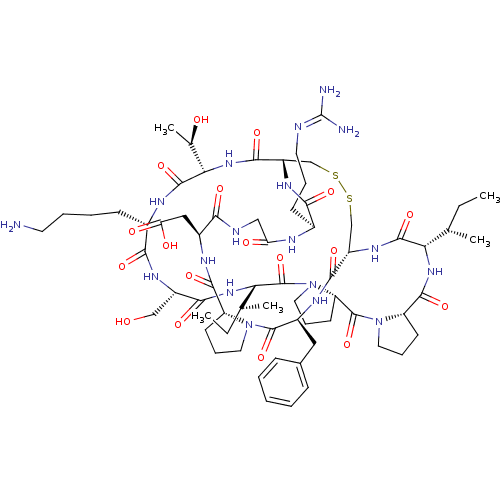
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21737
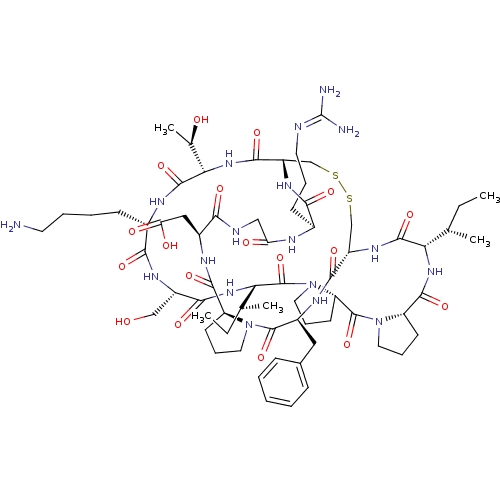
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288401
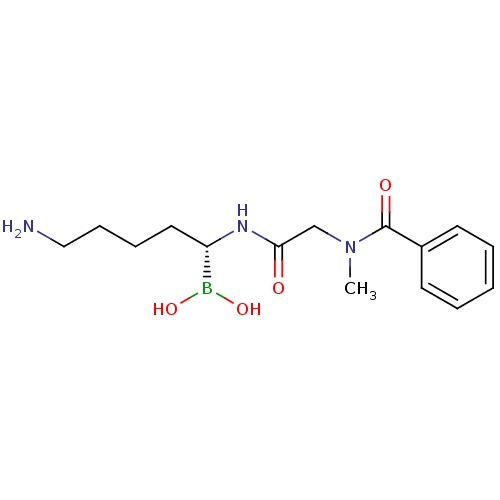
(CHEMBL317587 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show InChI InChI=1S/C15H24BN3O4/c1-19(15(21)12-7-3-2-4-8-12)11-14(20)18-13(16(22)23)9-5-6-10-17/h2-4,7-8,13,22-23H,5-6,9-11,17H2,1H3,(H,18,20)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of thrombin. |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288403

(CHEMBL330206 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CC(C)N(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C19H32BN3O4/c1-15(2)23(19(25)12-11-16-8-4-3-5-9-16)14-18(24)22-17(20(26)27)10-6-7-13-21/h3-5,8-9,15,17,26-27H,6-7,10-14,21H2,1-2H3,(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50288403

(CHEMBL330206 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES CC(C)N(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C19H32BN3O4/c1-15(2)23(19(25)12-11-16-8-4-3-5-9-16)14-18(24)22-17(20(26)27)10-6-7-13-21/h3-5,8-9,15,17,26-27H,6-7,10-14,21H2,1-2H3,(H,22,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50069174

(Boronate Ester analogue | CHEMBL317682 | N-[(5-Ami...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN3O4/c1-21(17(23)11-10-14-7-3-2-4-8-14)13-16(22)20-15(18(24)25)9-5-6-12-19/h2-4,7-8,15,24-25H,5-6,9-13,19H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288413

(CHEMBL101759 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C(=O)CCc1ccccc1)c1ccccc1)B(O)O Show InChI InChI=1S/C22H30BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1-6,9-12,20,29-30H,7-8,13-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50288407

(CHEMBL317137 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-8-17(20(26)27)22-18(24)14-23(16-10-11-16)19(25)12-9-15-6-2-1-3-7-15/h1-3,6-7,16-17,26-27H,4-5,8-14,21H2,(H,22,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21751
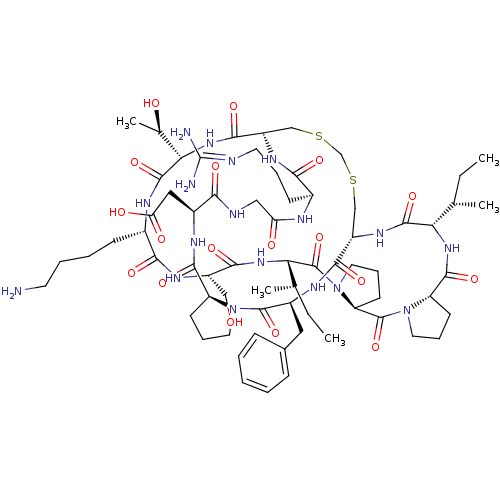
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50288409

(CHEMBL99309 | N-[(5-Amino-1-dihydroxyboranyl-penty...)Show SMILES NCCCC[C@H](NC(=O)CN(CCc1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C24H34BN3O4/c26-17-8-7-13-22(25(31)32)27-23(29)19-28(18-16-21-11-5-2-6-12-21)24(30)15-14-20-9-3-1-4-10-20/h1-6,9-12,22,31-32H,7-8,13-19,26H2,(H,27,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098555
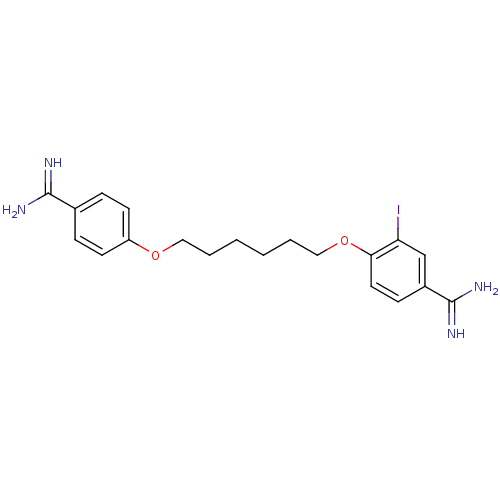
(4-[6-(4-Carbamimidoyl-phenoxy)-hexyloxy]-3-iodo-be...)Show InChI InChI=1S/C20H25IN4O2/c21-17-13-15(20(24)25)7-10-18(17)27-12-4-2-1-3-11-26-16-8-5-14(6-9-16)19(22)23/h5-10,13H,1-4,11-12H2,(H3,22,23)(H3,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288409

(CHEMBL99309 | N-[(5-Amino-1-dihydroxyboranyl-penty...)Show SMILES NCCCC[C@H](NC(=O)CN(CCc1ccccc1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C24H34BN3O4/c26-17-8-7-13-22(25(31)32)27-23(29)19-28(18-16-21-11-5-2-6-12-21)24(30)15-14-20-9-3-1-4-10-20/h1-6,9-12,22,31-32H,7-8,13-19,26H2,(H,27,29)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288414

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)B(O)O Show InChI InChI=1S/C21H33BN4O5/c1-15(27)24-17(14-16-8-3-2-4-9-16)21(29)26-13-7-10-18(26)20(28)25-19(22(30)31)11-5-6-12-23/h2-4,8-9,17-19,30-31H,5-7,10-14,23H2,1H3,(H,24,27)(H,25,28)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Bos taurus (Bovine)) | BDBM50288407

(CHEMBL317137 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)CCc1ccccc1)B(O)O Show InChI InChI=1S/C19H30BN3O4/c21-13-5-4-8-17(20(26)27)22-18(24)14-23(16-10-11-16)19(25)12-9-15-6-2-1-3-7-15/h1-3,6-7,16-17,26-27H,4-5,8-14,21H2,(H,22,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration necessary to double time for clot formation induced by bovine thrombin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098553
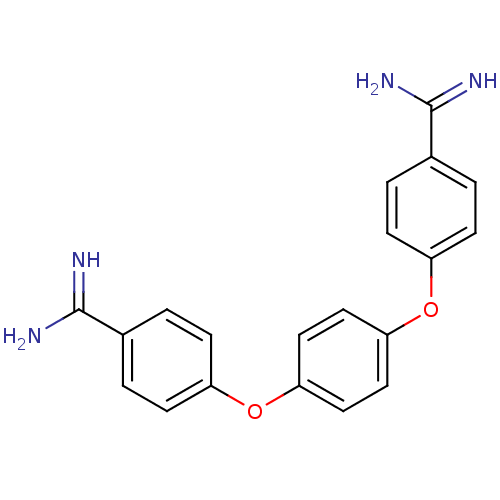
(4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...)Show SMILES NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)13-1-5-15(6-2-13)25-17-9-11-18(12-10-17)26-16-7-3-14(4-8-16)20(23)24/h1-12H,(H3,21,22)(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50015234
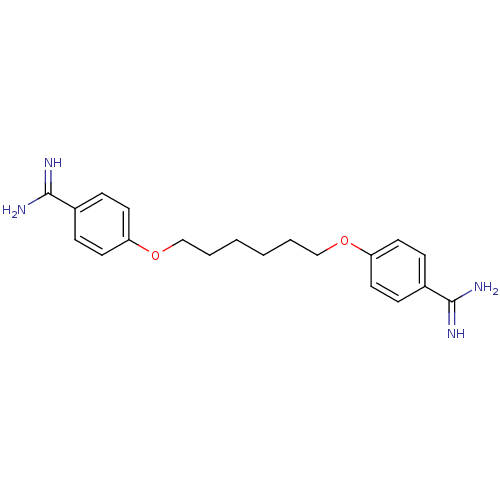
(4,4''[1,6-HEXANEDIYLBIS(OXY)]BISBENZENECARBOXIMIDA...)Show InChI InChI=1S/C20H26N4O2/c21-19(22)15-5-9-17(10-6-15)25-13-3-1-2-4-14-26-18-11-7-16(8-12-18)20(23)24/h5-12H,1-4,13-14H2,(H3,21,22)(H3,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Thrombin |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50288413

(CHEMBL101759 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C(=O)CCc1ccccc1)c1ccccc1)B(O)O Show InChI InChI=1S/C22H30BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1-6,9-12,20,29-30H,7-8,13-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50069174

(Boronate Ester analogue | CHEMBL317682 | N-[(5-Ami...)Show SMILES CN(CC(=O)N[C@@H](CCCCN)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN3O4/c1-21(17(23)11-10-14-7-3-2-4-8-14)13-16(22)20-15(18(24)25)9-5-6-12-19/h2-4,7-8,15,24-25H,5-6,9-13,19H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro for inhibition of plasmin |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data