| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Suppressor of tumorigenicity 14 protein |
|---|
| Ligand | BDBM50421510 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_105805 |
|---|
| Ki | 0.920000±n/a nM |
|---|
| Citation |  Long, YQ; Lee, SL; Lin, CY; Enyedy, IJ; Wang, S; Li, P; Dickson, RB; Roller, PP Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett11:2515-9 (2001) [PubMed] Long, YQ; Lee, SL; Lin, CY; Enyedy, IJ; Wang, S; Li, P; Dickson, RB; Roller, PP Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett11:2515-9 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Suppressor of tumorigenicity 14 protein |
|---|
| Name: | Suppressor of tumorigenicity 14 protein |
|---|
| Synonyms: | Epithin | Hepatocyte growth factor activator/Serine protease hepsin/Suppressor of tumorigenicity 14 protein | MT-SP1 | Membrane-type serine protease 1 | PRSS14 | Prostamin | SNC19 | ST14 | ST14_HUMAN | Serine protease TADG-15 | Suppressor of tumorigenicity 14 protein | Suppressor of tumorigenicity protein 14 | TADG15 |
|---|
| Type: | Single-pass type II membrane protein |
|---|
| Mol. Mass.: | 94769.23 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q9Y5Y6 |
|---|
| Residue: | 855 |
|---|
| Sequence: | MGSDRARKGGGGPKDFGAGLKYNSRHEKVNGLEEGVEFLPVNNVKKVEKHGPGRWVVLAA
VLIGLLLVLLGIGFLVWHLQYRDVRVQKVFNGYMRITNENFVDAYENSNSTEFVSLASKV
KDALKLLYSGVPFLGPYHKESAVTAFSEGSVIAYYWSEFSIPQHLVEEAERVMAEERVVM
LPPRARSLKSFVVTSVVAFPTDSKTVQRTQDNSCSFGLHARGVELMRFTTPGFPDSPYPA
HARCQWALRGDADSVLSLTFRSFDLASCDERGSDLVTVYNTLSPMEPHALVQLCGTYPPS
YNLTFHSSQNVLLITLITNTERRHPGFEATFFQLPRMSSCGGRLRKAQGTFNSPYYPGHY
PPNIDCTWNIEVPNNQHVKVRFKFFYLLEPGVPAGTCPKDYVEINGEKYCGERSQFVVTS
NSNKITVRFHSDQSYTDTGFLAEYLSYDSSDPCPGQFTCRTGRCIRKELRCDGWADCTDH
SDELNCSCDAGHQFTCKNKFCKPLFWVCDSVNDCGDNSDEQGCSCPAQTFRCSNGKCLSK
SQQCNGKDDCGDGSDEASCPKVNVVTCTKHTYRCLNGLCLSKGNPECDGKEDCSDGSDEK
DCDCGLRSFTRQARVVGGTDADEGEWPWQVSLHALGQGHICGASLISPNWLVSAAHCYID
DRGFRYSDPTQWTAFLGLHDQSQRSAPGVQERRLKRIISHPFFNDFTFDYDIALLELEKP
AEYSSMVRPICLPDASHVFPAGKAIWVTGWGHTQYGGTGALILQKGEIRVINQTTCENLL
PQQITPRMMCVGFLSGGVDSCQGDSGGPLSSVEADGRIFQAGVVSWGDGCAQRNKPGVYT
RLPLFRDWIKENTGV
|
|
|
|---|
| BDBM50421510 |
|---|
| n/a |
|---|
| Name | BDBM50421510 |
|---|
| Synonyms: | CHEMBL239127 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C67H104N18O18S2 |
|---|
| Mol. Mass. | 1513.782 |
|---|
| SMILES | [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |
|---|
| Structure | 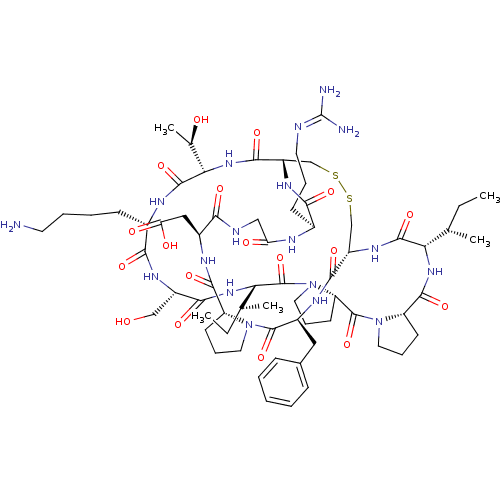
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Long, YQ; Lee, SL; Lin, CY; Enyedy, IJ; Wang, S; Li, P; Dickson, RB; Roller, PP Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett11:2515-9 (2001) [PubMed]
Long, YQ; Lee, SL; Lin, CY; Enyedy, IJ; Wang, S; Li, P; Dickson, RB; Roller, PP Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett11:2515-9 (2001) [PubMed]