

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypsin (Homo sapiens (Human)) | BDBM50421510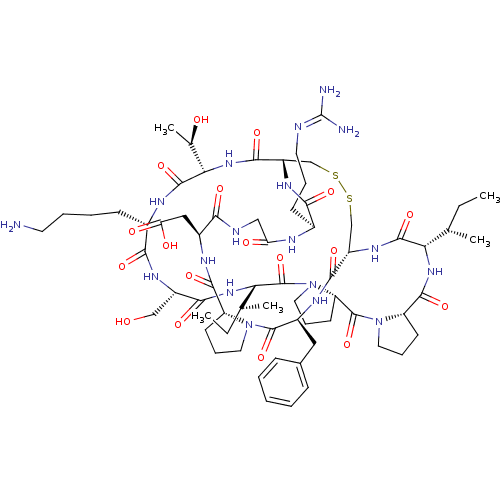 (CHEMBL239127) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of Sunflower beta-trypsin | Bioorg Med Chem Lett 11: 2515-9 (2001) BindingDB Entry DOI: 10.7270/Q2TH8M0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50421510 (CHEMBL239127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of Matriptase from human breast cancer cells | Bioorg Med Chem Lett 11: 2515-9 (2001) BindingDB Entry DOI: 10.7270/Q2TH8M0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-3 (Homo sapiens (Human)) | BDBM50421510 (CHEMBL239127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of bovine beta trypsin | Bioorg Med Chem Lett 11: 2515-9 (2001) BindingDB Entry DOI: 10.7270/Q2TH8M0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50274428 (CHEMBL4127538) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Binding affinity to human CCR2 | Bioorg Med Chem 26: 3559-3572 (2018) Article DOI: 10.1016/j.bmc.2018.05.027 BindingDB Entry DOI: 10.7270/Q2H41TZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116661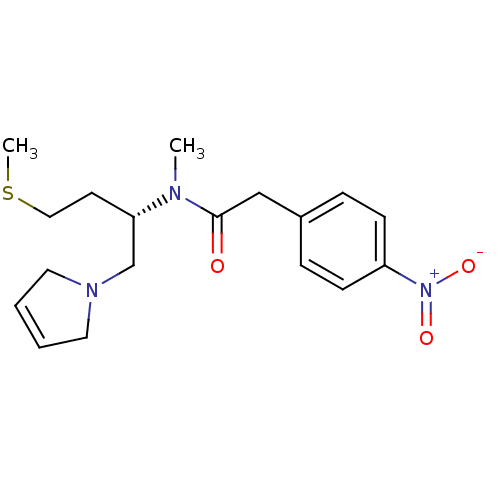 (CHEMBL75668 | N-[(S)-1-(2,5-Dihydro-pyrrol-1-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine in cloned rat Opioid receptor kappa 1 expressed in Sf9 insect cells | Bioorg Med Chem Lett 12: 2287-90 (2002) BindingDB Entry DOI: 10.7270/Q2QV3KTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116659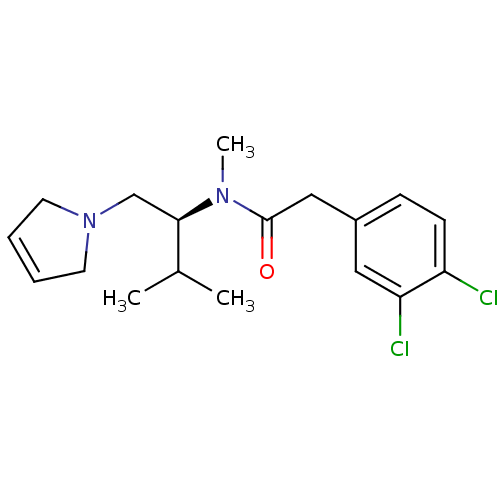 (2-(3,4-Dichloro-phenyl)-N-[(S)-1-(2,5-dihydro-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine in cloned rat Opioid receptor kappa 1 expressed in Sf9 insect cells | Bioorg Med Chem Lett 12: 2287-90 (2002) BindingDB Entry DOI: 10.7270/Q2QV3KTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50274429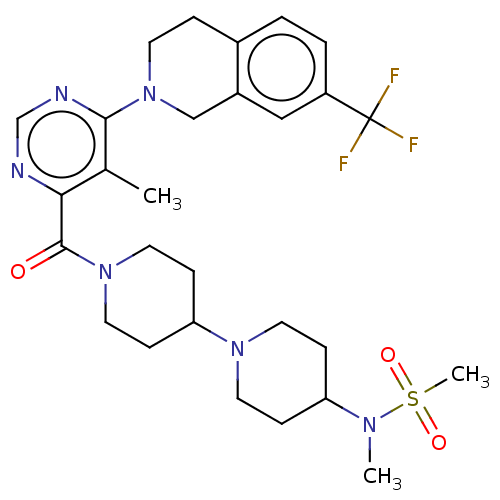 (CHEMBL4126162) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Binding affinity to human CCR2 | Bioorg Med Chem 26: 3559-3572 (2018) Article DOI: 10.1016/j.bmc.2018.05.027 BindingDB Entry DOI: 10.7270/Q2H41TZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50274430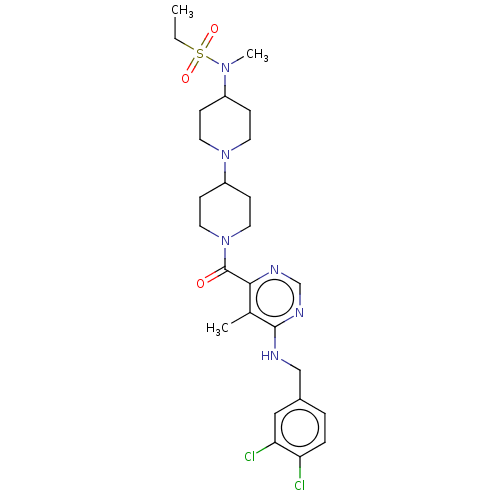 (CHEMBL4127496) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Binding affinity to human CCR2 | Bioorg Med Chem 26: 3559-3572 (2018) Article DOI: 10.1016/j.bmc.2018.05.027 BindingDB Entry DOI: 10.7270/Q2H41TZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50404040 (CHEMBL5288934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of Ras Farnesyltransferase enzyme from pig brain | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50045060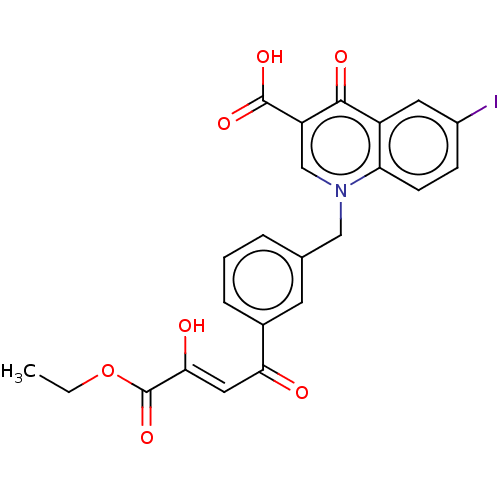 (CHEMBL3309789) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of PTP1B (unknown origin) using pNPP as substrate by Lineweaver-Burk plot | Bioorg Med Chem 22: 3670-83 (2014) Article DOI: 10.1016/j.bmc.2014.05.028 BindingDB Entry DOI: 10.7270/Q2G44RX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50107130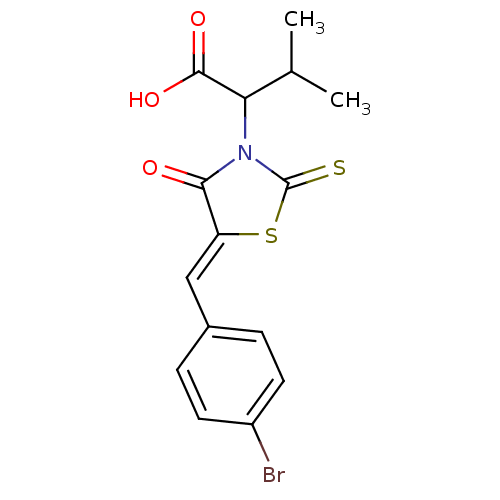 ((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibitory activity against Bcl-2 | J Med Chem 44: 4313-24 (2001) BindingDB Entry DOI: 10.7270/Q2JW8FKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50421510 (CHEMBL239127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of bovine Thrombin | Bioorg Med Chem Lett 11: 2515-9 (2001) BindingDB Entry DOI: 10.7270/Q2TH8M0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase plasminogen activator surface receptor (Homo sapiens (Human)) | BDBM50421510 (CHEMBL239127) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was tested for microPa) Urokinase-type plasminogen activator from human urine | Bioorg Med Chem Lett 11: 2515-9 (2001) BindingDB Entry DOI: 10.7270/Q2TH8M0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327846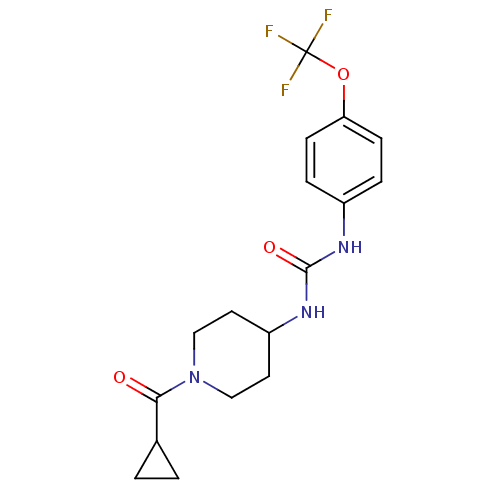 (1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase (unknown origin) | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496759 (CHEMBL3222130) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496754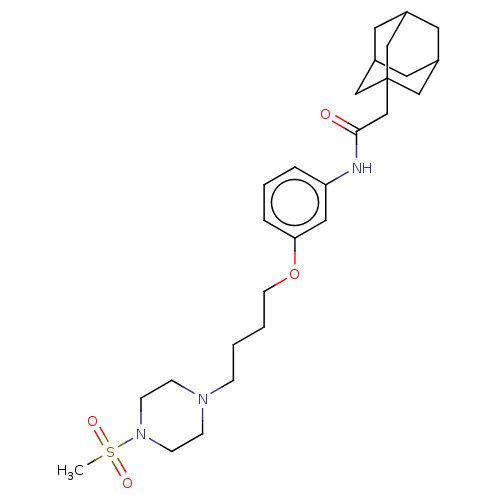 (CHEMBL3222131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496748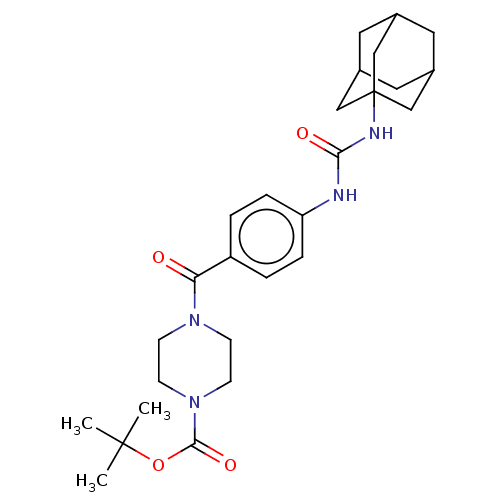 (CHEMBL3222118) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50404041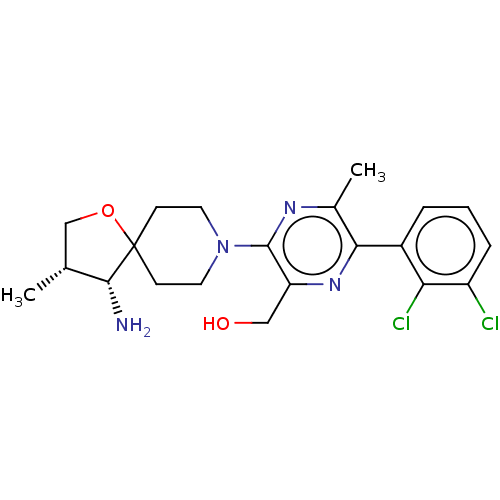 (CHEMBL5287751) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.583 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496753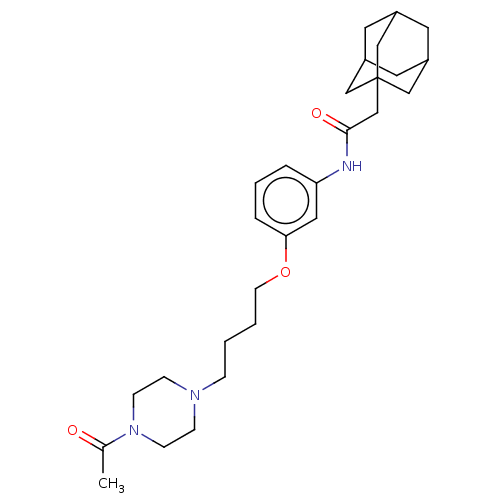 (CHEMBL3222129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 (unknown origin) expressed in CHO cells by scintillation counting analysis | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496760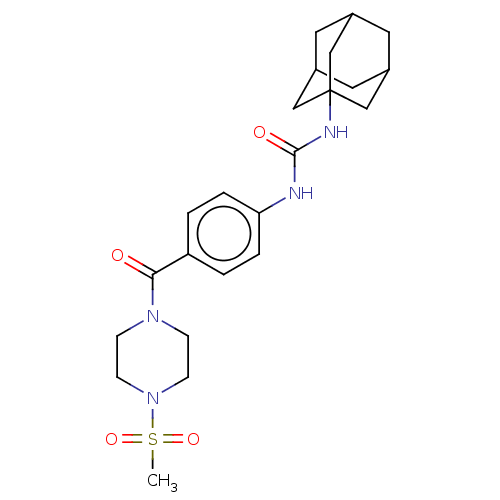 (CHEMBL3222119) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458294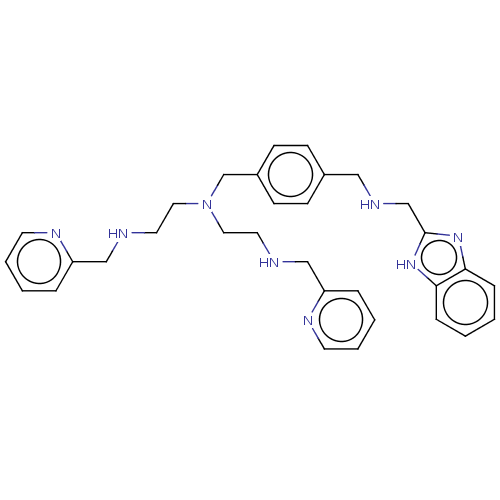 (CHEMBL4210165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458281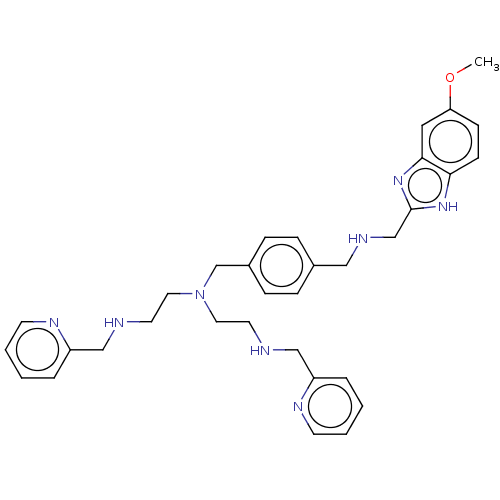 (CHEMBL4217502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496757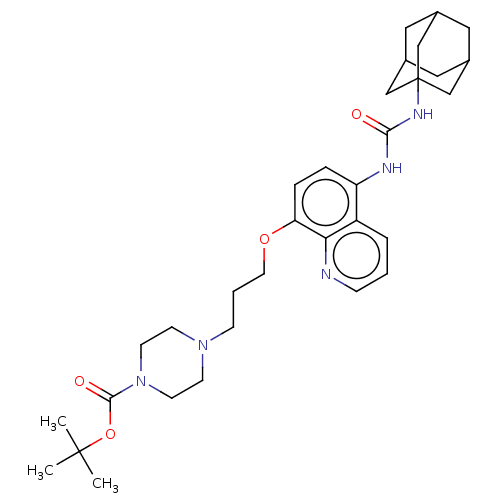 (CHEMBL3222121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50074167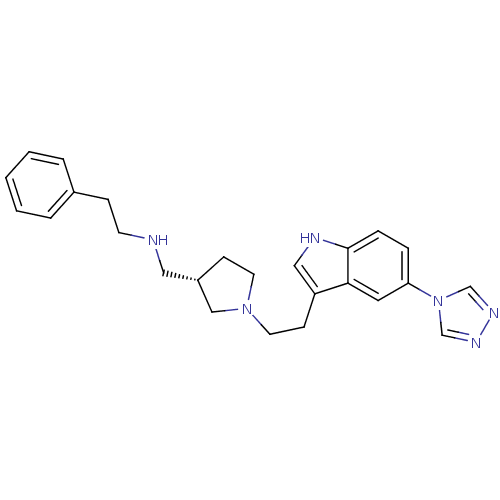 (CHEMBL348815 | Phenethyl-{(S)-1-[2-(5-[1,2,4]triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50074178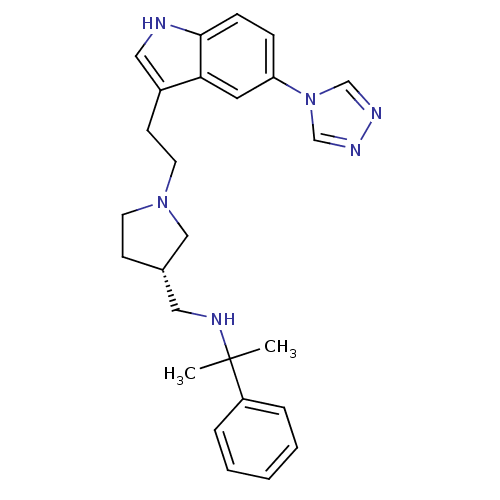 ((1-Methyl-1-phenyl-ethyl)-{(S)-1-[2-(5-[1,2,4]tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458290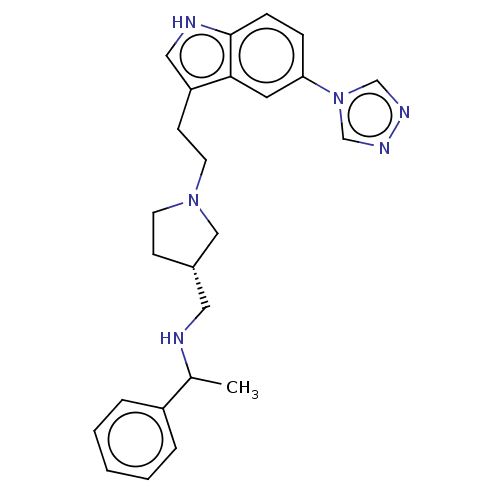 (CHEMBL4215051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458283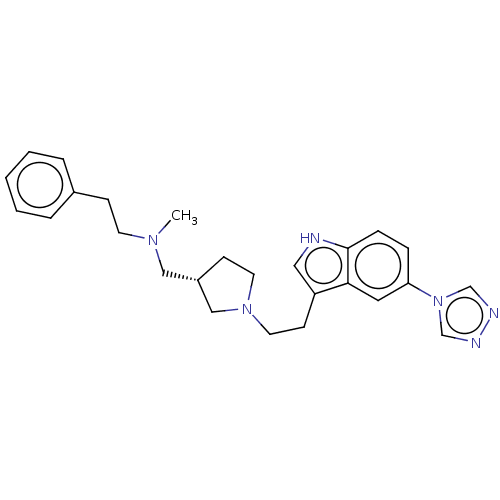 (CHEMBL4212661) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496746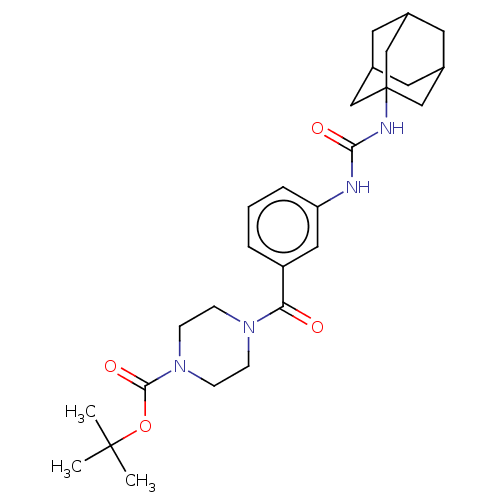 (CHEMBL3222115) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496747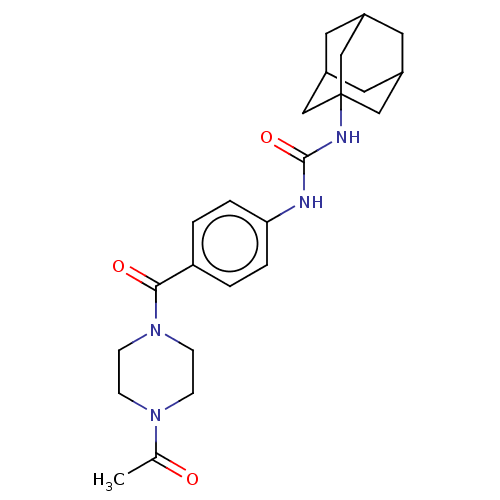 (CHEMBL3222117) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50200480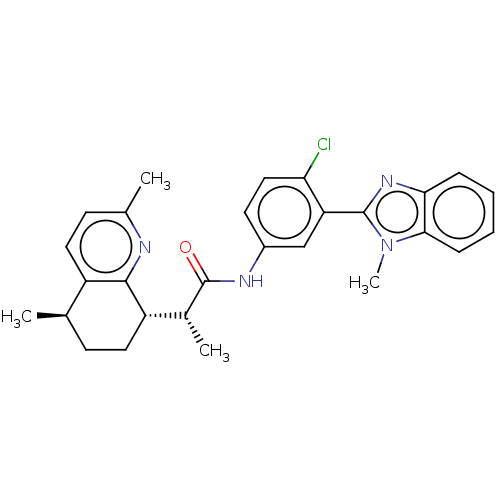 (CHEMBL3965352) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signalling pathway in mouse Shh Light2 cells by Gli-luciferase reporter gene assay | J Med Chem 59: 11050-11068 (2016) Article DOI: 10.1021/acs.jmedchem.6b01247 BindingDB Entry DOI: 10.7270/Q2TQ63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458288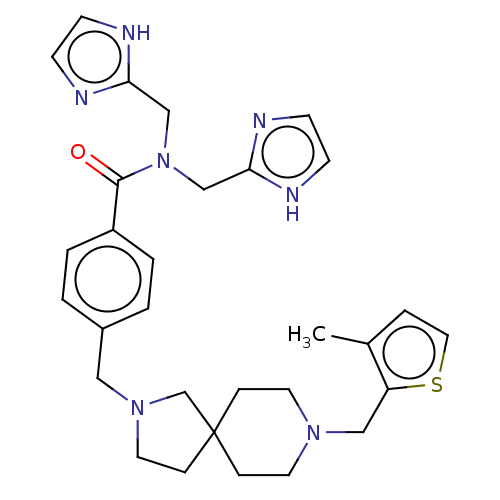 (CHEMBL4218006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR2 (unknown origin) expressed in CHOK1 cells co-expressing Ga16 assessed as inhibition of CCL2 induced intracellular Ca2+ mo... | Bioorg Med Chem 26: 3559-3572 (2018) Article DOI: 10.1016/j.bmc.2018.05.027 BindingDB Entry DOI: 10.7270/Q2H41TZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458286 (CHEMBL2367715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human MT4 cells after 2 hrs by scintillation counting analysis | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50314723 (CHEMBL1090041 | endo-(S)-N-(4-fluorobenzyl)-4-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in CHO cells assessed as inhibition of RANTES-induced [32S]GTPgammaS binding | Bioorg Med Chem Lett 20: 2219-23 (2010) Article DOI: 10.1016/j.bmcl.2010.02.023 BindingDB Entry DOI: 10.7270/Q2C53M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458287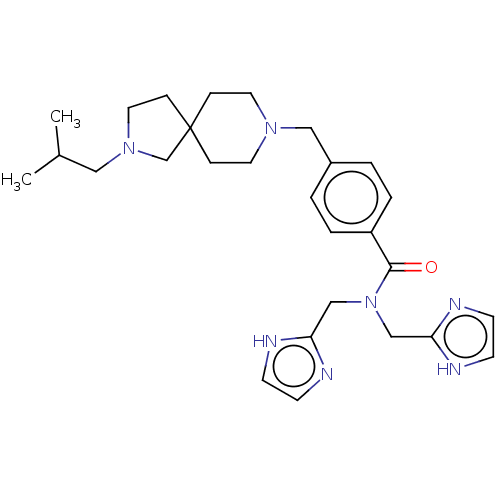 (CHEMBL4213081) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496758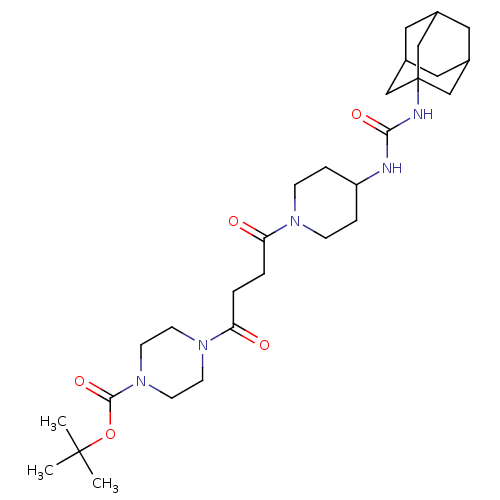 (CHEMBL3222124) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458297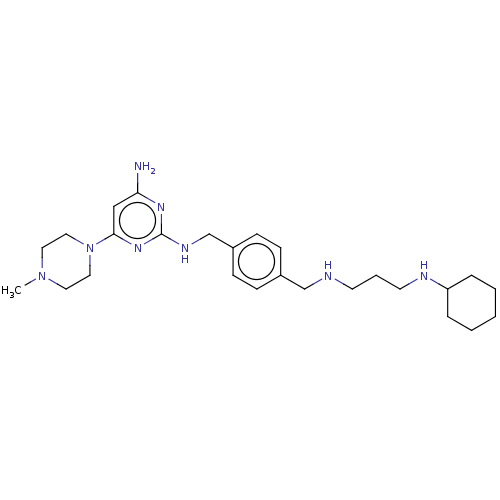 (CHEMBL4213087) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458292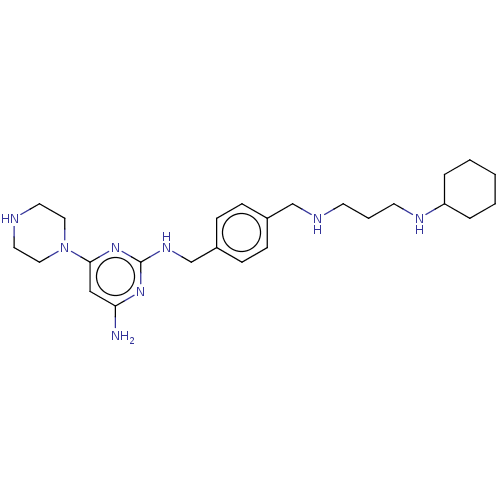 (CHEMBL4218011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458293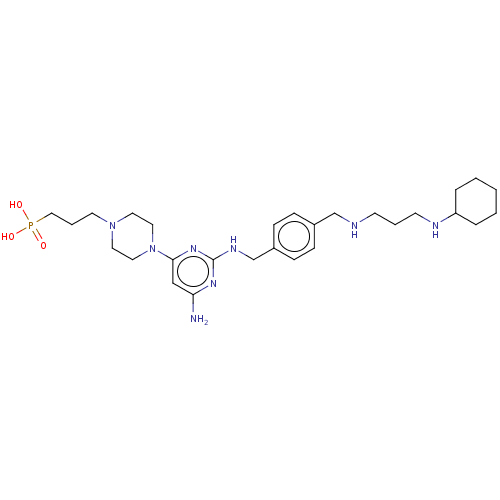 (CHEMBL4205801) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50080122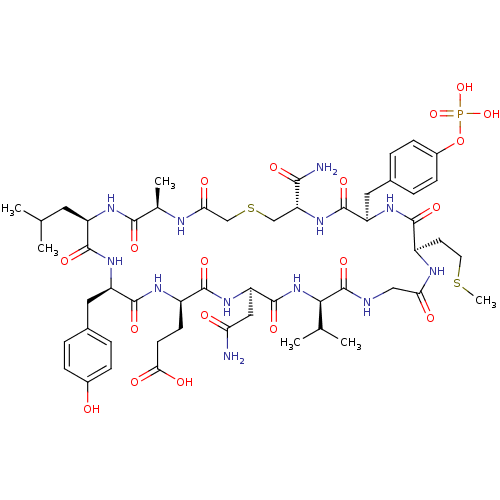 (CHEMBL407859 | Cyclo peptide analogue) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Growth factor receptor bound protein 2 | Bioorg Med Chem Lett 9: 2267-72 (1999) BindingDB Entry DOI: 10.7270/Q2VH5N1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458279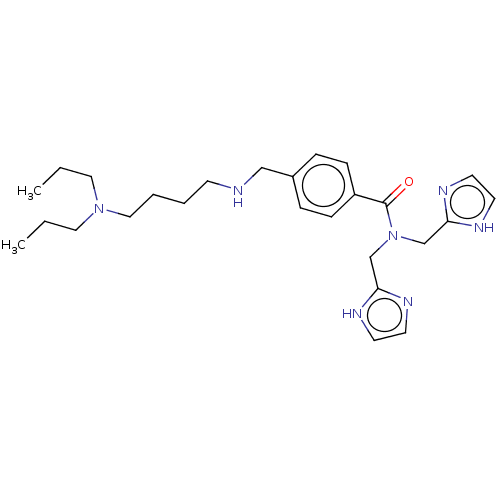 (CHEMBL4206706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of SDF-1 binding to CXCR4 (unknown origin) | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50155310 (CHEMBL3779982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50249522 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signalling pathway in mouse Shh Light2 cells by Gli-luciferase reporter gene assay | J Med Chem 59: 11050-11068 (2016) Article DOI: 10.1021/acs.jmedchem.6b01247 BindingDB Entry DOI: 10.7270/Q2TQ63G3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496756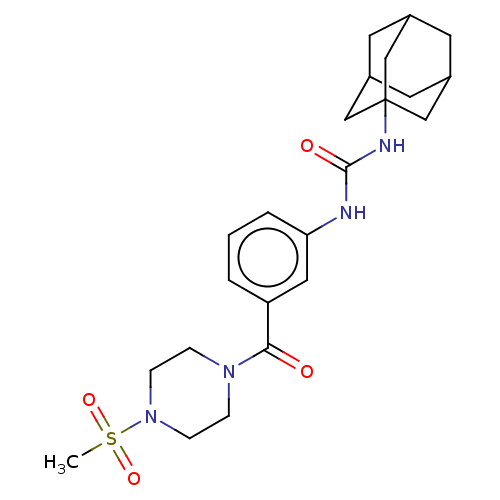 (CHEMBL3222116) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496751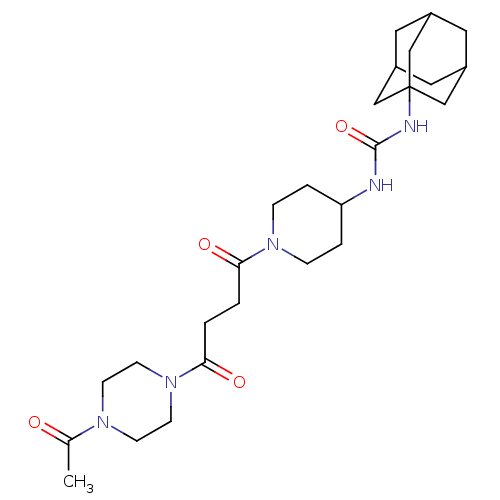 (CHEMBL3222123) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496745 (CHEMBL3222114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50200484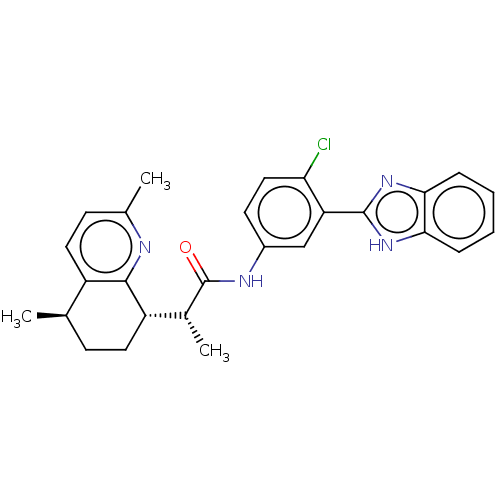 (CHEMBL3960082) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signalling pathway in mouse Shh Light2 cells by Gli-luciferase reporter gene assay | J Med Chem 59: 11050-11068 (2016) Article DOI: 10.1021/acs.jmedchem.6b01247 BindingDB Entry DOI: 10.7270/Q2TQ63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50147397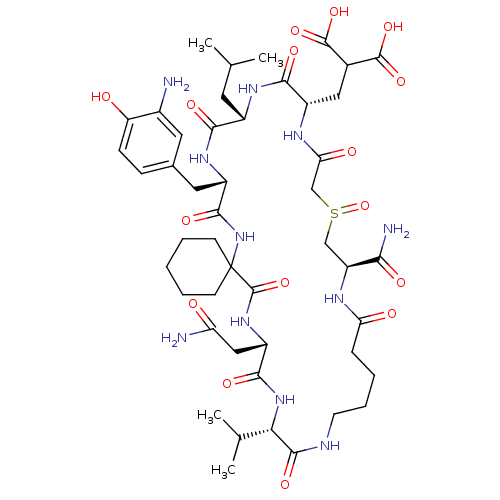 (CHEMBL444897 | Hexapeptide derivative) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Competitive binding affinity against Growth factor receptor bound protein 2 was assessed using SHC(pTyr-317) phospopeptide in biacore surface plasmon... | Bioorg Med Chem Lett 14: 3205-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.103 BindingDB Entry DOI: 10.7270/Q28K78J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50200482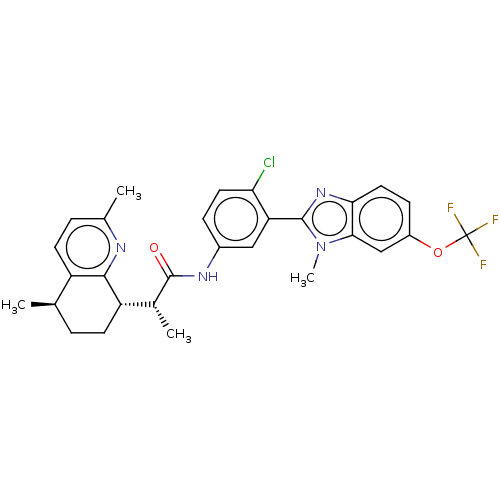 (CHEMBL3978393) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signalling pathway in mouse Shh Light2 cells by Gli-luciferase reporter gene assay | J Med Chem 59: 11050-11068 (2016) Article DOI: 10.1021/acs.jmedchem.6b01247 BindingDB Entry DOI: 10.7270/Q2TQ63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 839 total ) | Next | Last >> |