 Found 47 hits with Last Name = 'dickson' and Initial = 'rb'
Found 47 hits with Last Name = 'dickson' and Initial = 'rb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypsin
(Homo sapiens (Human)) | BDBM50421510
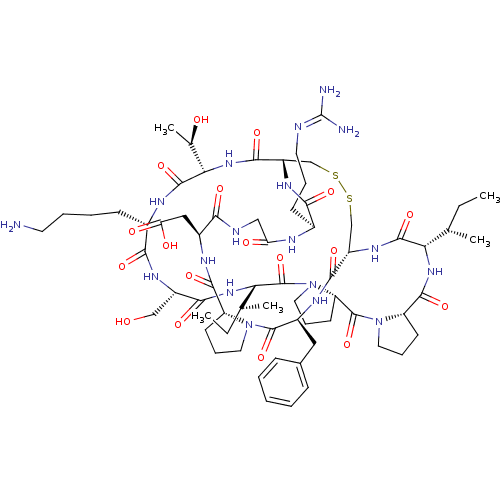
(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Sunflower beta-trypsin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Matriptase from human breast cancer cells |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Trypsin-3
(Homo sapiens (Human)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of bovine beta trypsin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21737
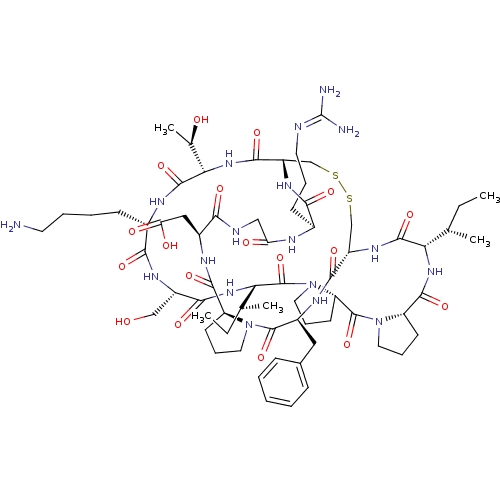
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21751
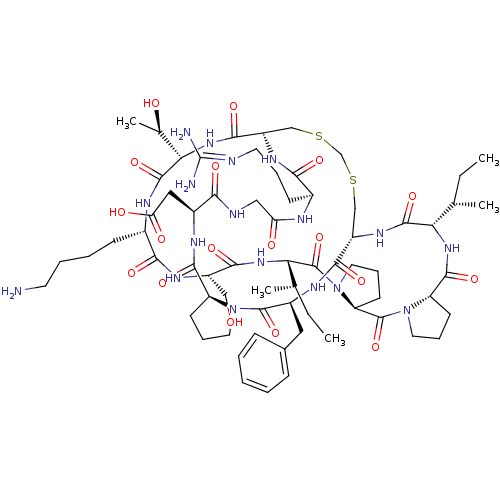
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098555
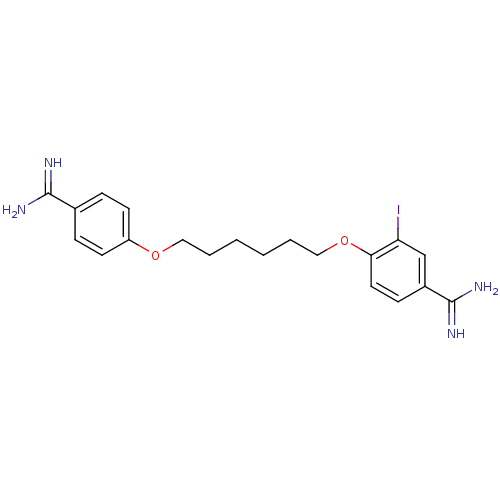
(4-[6-(4-Carbamimidoyl-phenoxy)-hexyloxy]-3-iodo-be...)Show InChI InChI=1S/C20H25IN4O2/c21-17-13-15(20(24)25)7-10-18(17)27-12-4-2-1-3-11-26-16-8-5-14(6-9-16)19(22)23/h5-10,13H,1-4,11-12H2,(H3,22,23)(H3,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098553
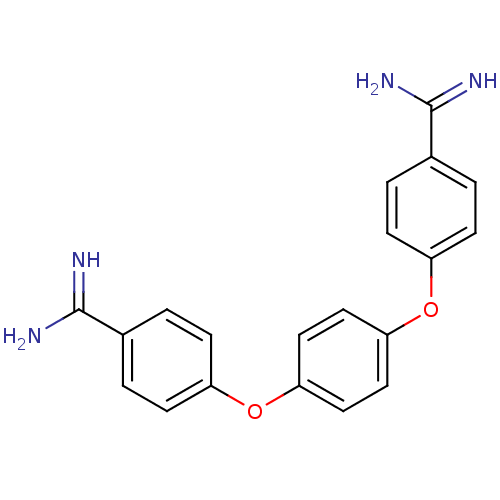
(4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...)Show SMILES NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)13-1-5-15(6-2-13)25-17-9-11-18(12-10-17)26-16-7-3-14(4-8-16)20(23)24/h1-12H,(H3,21,22)(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50015234
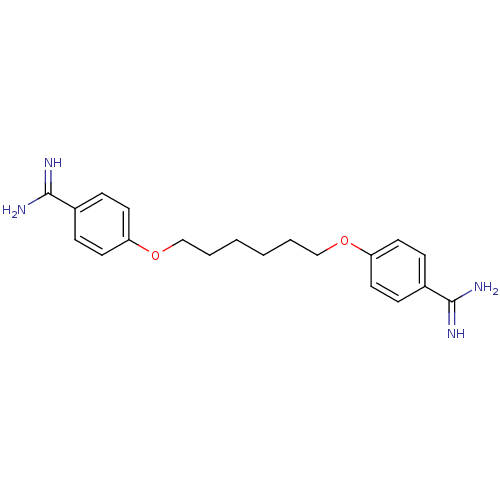
(4,4''[1,6-HEXANEDIYLBIS(OXY)]BISBENZENECARBOXIMIDA...)Show InChI InChI=1S/C20H26N4O2/c21-19(22)15-5-9-17(10-6-15)25-13-3-1-2-4-14-26-18-11-7-16(8-12-18)20(23)24/h5-12H,1-4,13-14H2,(H3,21,22)(H3,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Thrombin |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21750
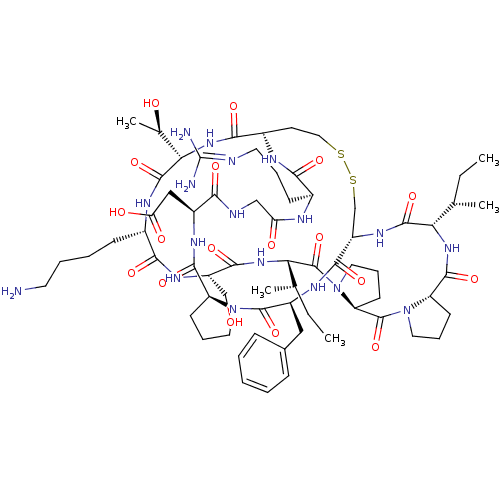
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31S,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-46-35-106-105-30-24-42(75-56(93)40(20-13-26-72-68(70)71)74-50(89)33-73-55(92)43(32-51(90)91)77-61(98)47-21-14-27-84(47)65(102)44(78-60(46)97)31-39-17-9-8-10-18-39)58(95)83-54(38(5)88)64(101)76-41(19-11-12-25-69)57(94)79-45(34-87)59(96)82-53(37(4)7-2)67(104)86-29-16-23-49(86)66(103)85-28-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,93)(H,76,101)(H,77,98)(H,78,97)(H,79,94)(H,80,100)(H,81,99)(H,82,96)(H,83,95)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098554
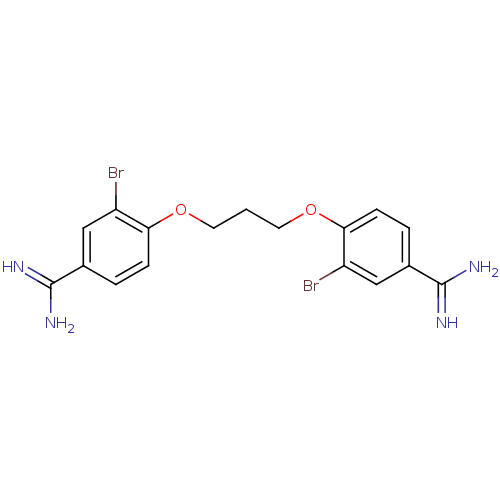
(4-(3-{4-[amino(imino)methyl]-2-bromophenoxy}propox...)Show SMILES NC(=N)c1ccc(OCCCOc2ccc(cc2Br)C(N)=N)c(Br)c1 Show InChI InChI=1S/C17H18Br2N4O2/c18-12-8-10(16(20)21)2-4-14(12)24-6-1-7-25-15-5-3-11(17(22)23)9-13(15)19/h2-5,8-9H,1,6-7H2,(H3,20,21)(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 535 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098555

(4-[6-(4-Carbamimidoyl-phenoxy)-hexyloxy]-3-iodo-be...)Show InChI InChI=1S/C20H25IN4O2/c21-17-13-15(20(24)25)7-10-18(17)27-12-4-2-1-3-11-26-16-8-5-14(6-9-16)19(22)23/h5-10,13H,1-4,11-12H2,(H3,22,23)(H3,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 796 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Thrombin |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21749
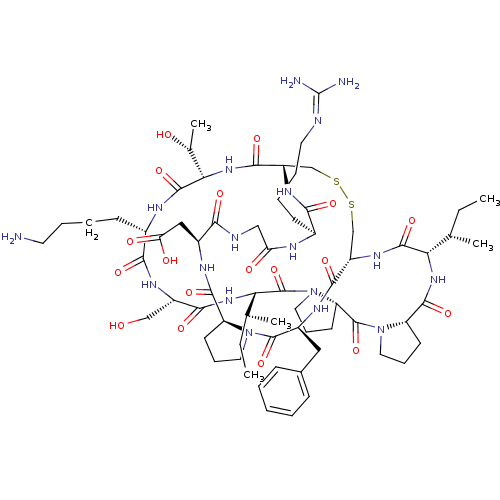
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21739
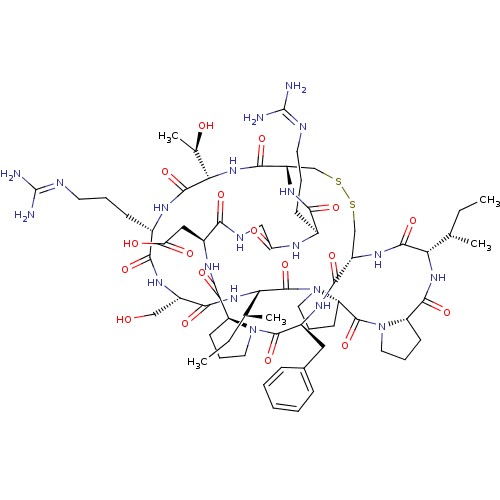
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21748
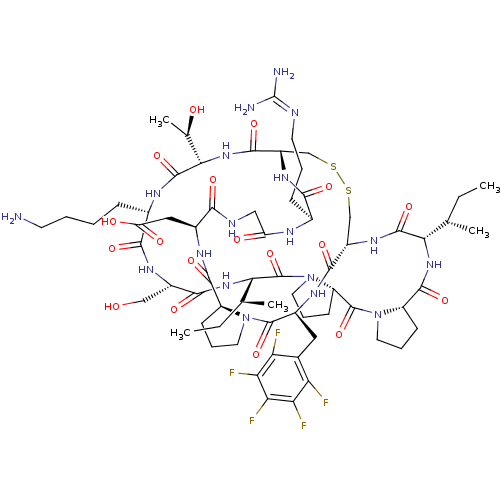
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | -34.2 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50015234

(4,4''[1,6-HEXANEDIYLBIS(OXY)]BISBENZENECARBOXIMIDA...)Show InChI InChI=1S/C20H26N4O2/c21-19(22)15-5-9-17(10-6-15)25-13-3-1-2-4-14-26-18-11-7-16(8-12-18)20(23)24/h5-12H,1-4,13-14H2,(H3,21,22)(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 924 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098554

(4-(3-{4-[amino(imino)methyl]-2-bromophenoxy}propox...)Show SMILES NC(=N)c1ccc(OCCCOc2ccc(cc2Br)C(N)=N)c(Br)c1 Show InChI InChI=1S/C17H18Br2N4O2/c18-12-8-10(16(20)21)2-4-14(12)24-6-1-7-25-15-5-3-11(17(22)23)9-13(15)19/h2-5,8-9H,1,6-7H2,(H3,20,21)(H3,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 946 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Thrombin |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM45440
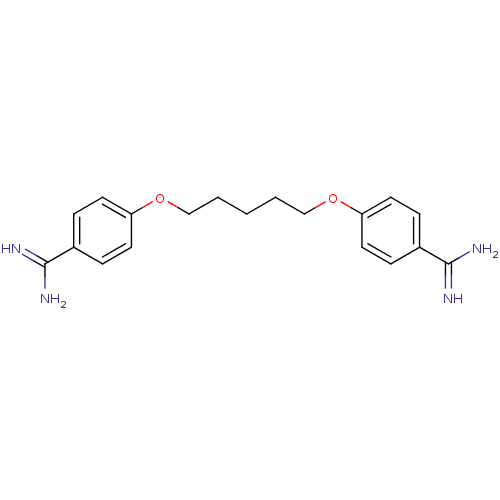
(4-[5-(4-amidinophenoxy)pentoxy]benzamidine;2-hydro...)Show InChI InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098554

(4-(3-{4-[amino(imino)methyl]-2-bromophenoxy}propox...)Show SMILES NC(=N)c1ccc(OCCCOc2ccc(cc2Br)C(N)=N)c(Br)c1 Show InChI InChI=1S/C17H18Br2N4O2/c18-12-8-10(16(20)21)2-4-14(12)24-6-1-7-25-15-5-3-11(17(22)23)9-13(15)19/h2-5,8-9H,1,6-7H2,(H3,20,21)(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa) |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098553

(4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...)Show SMILES NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)13-1-5-15(6-2-13)25-17-9-11-18(12-10-17)26-16-7-3-14(4-8-16)20(23)24/h1-12H,(H3,21,22)(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa) |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098555

(4-[6-(4-Carbamimidoyl-phenoxy)-hexyloxy]-3-iodo-be...)Show InChI InChI=1S/C20H25IN4O2/c21-17-13-15(20(24)25)7-10-18(17)27-12-4-2-1-3-11-26-16-8-5-14(6-9-16)19(22)23/h5-10,13H,1-4,11-12H2,(H3,22,23)(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa) |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21745
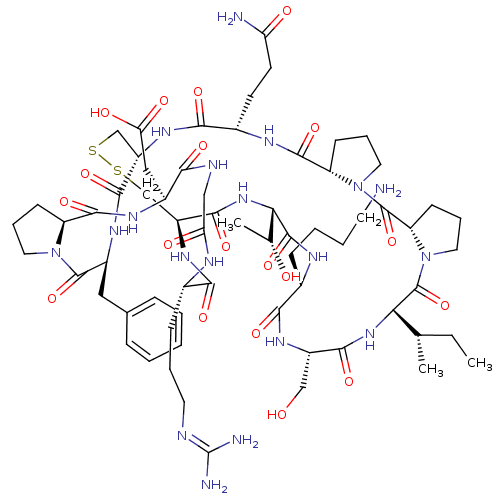
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098553

(4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...)Show SMILES NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)13-1-5-15(6-2-13)25-17-9-11-18(12-10-17)26-16-7-3-14(4-8-16)20(23)24/h1-12H,(H3,21,22)(H3,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Thrombin |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098552

(1,5-Bis-[(4-amino(imino)methyl)phenyldiazeno]-anth...)Show SMILES NC(=N)c1ccc(cc1)N=Nc1c(O)ccc2cc3c(N=Nc4ccc(cc4)C(N)=N)c(O)ccc3cc12 |w:9.9,21.22| Show InChI InChI=1S/C28H22N8O2/c29-27(30)15-1-7-19(8-2-15)33-35-25-21-13-18-6-12-24(38)26(22(18)14-17(21)5-11-23(25)37)36-34-20-9-3-16(4-10-20)28(31)32/h1-14,37-38H,(H3,29,30)(H3,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21739

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21737

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of bovine Thrombin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21749

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.63E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21751

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098556
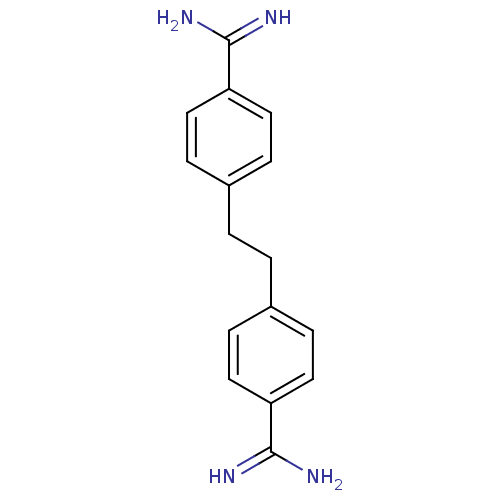
(4-(2-{4-[amino(imino)methyl]phenyl}ethyl)benzeneca...)Show InChI InChI=1S/C16H18N4/c17-15(18)13-7-3-11(4-8-13)1-2-12-5-9-14(10-6-12)16(19)20/h3-10H,1-2H2,(H3,17,18)(H3,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Matriptase |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50015234

(4,4''[1,6-HEXANEDIYLBIS(OXY)]BISBENZENECARBOXIMIDA...)Show InChI InChI=1S/C20H26N4O2/c21-19(22)15-5-9-17(10-6-15)25-13-3-1-2-4-14-26-18-11-7-16(8-12-18)20(23)24/h5-12H,1-4,13-14H2,(H3,21,22)(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa) |
J Med Chem 44: 1349-55 (2001)
BindingDB Entry DOI: 10.7270/Q2057F62 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21742
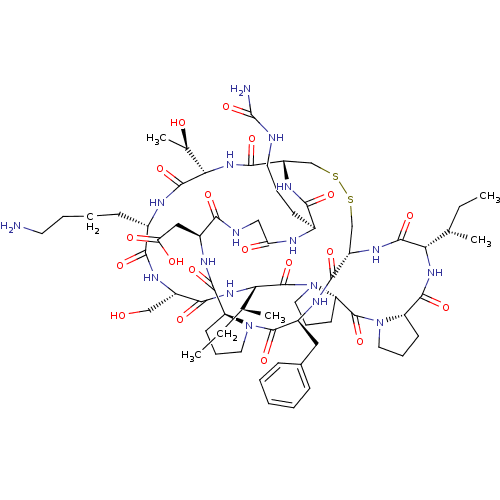
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.25E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21743
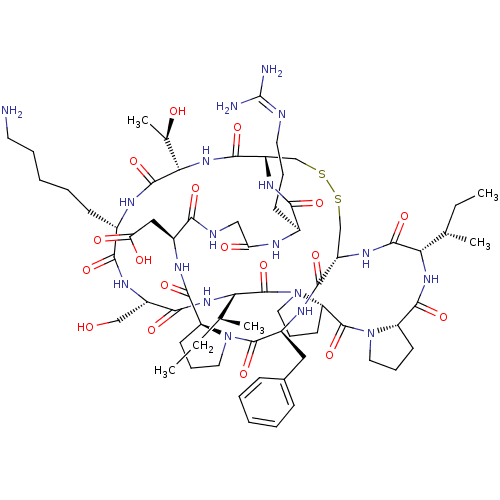
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21748

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.36E+4 | -25.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21744
(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.04E+4 | -24.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21743

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.56E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | -22.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21744

(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+5 | -22.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21740
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C66H102N18O18S2/c1-6-34(3)50-61(98)78-43-32-103-104-33-44(77-54(91)38(19-12-24-70-66(68)69)72-48(87)30-71-53(90)40(29-49(88)89)74-59(96)45-20-13-25-82(45)63(100)41(75-57(43)94)28-37-16-9-8-10-17-37)58(95)81-52(36(5)86)62(99)73-39(18-11-23-67)55(92)76-42(31-85)56(93)80-51(35(4)7-2)65(102)84-27-15-22-47(84)64(101)83-26-14-21-46(83)60(97)79-50/h8-10,16-17,34-36,38-47,50-52,85-86H,6-7,11-15,18-33,67H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H4,68,69,70)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+5 | >-20.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50421510

(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for microPa) Urokinase-type plasminogen activator from human urine |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21742

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.27E+5 | -17.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21745

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21740

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C66H102N18O18S2/c1-6-34(3)50-61(98)78-43-32-103-104-33-44(77-54(91)38(19-12-24-70-66(68)69)72-48(87)30-71-53(90)40(29-49(88)89)74-59(96)45-20-13-25-82(45)63(100)41(75-57(43)94)28-37-16-9-8-10-17-37)58(95)81-52(36(5)86)62(99)73-39(18-11-23-67)55(92)76-42(31-85)56(93)80-51(35(4)7-2)65(102)84-27-15-22-47(84)64(101)83-26-14-21-46(83)60(97)79-50/h8-10,16-17,34-36,38-47,50-52,85-86H,6-7,11-15,18-33,67H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H4,68,69,70)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data














































