| Synonyms: | PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K |
|---|
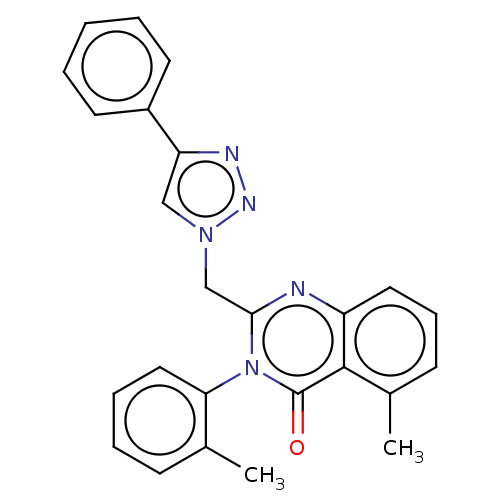
| SMILES | Cc1ccccc1-n1c(Cn2cc(nn2)-c2ccccc2)nc2cccc(C)c2c1=O |(24.56,-20.94,;25.89,-20.17,;25.89,-18.63,;27.22,-17.86,;28.56,-18.63,;28.56,-20.17,;27.22,-20.94,;27.22,-22.48,;25.89,-23.25,;24.56,-22.48,;23.22,-23.25,;21.82,-22.62,;20.79,-23.77,;21.56,-25.1,;23.06,-24.78,;19.25,-23.74,;18.45,-25.05,;16.91,-25.02,;16.17,-23.68,;16.96,-22.36,;18.5,-22.39,;25.89,-24.79,;27.22,-25.56,;27.22,-27.1,;28.56,-27.87,;29.89,-27.1,;29.89,-25.56,;31.23,-24.79,;28.56,-24.79,;28.56,-23.25,;29.89,-22.48,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Srinivas, M; Singh Pathania, A; Mahajan, P; Verma, PK; Chobe, SS; Malik, FA; Nargotra, A; Vishwakarma, RA; Sawant, SD Design and synthesis of 1,4-substituted 1H-1,2,3-triazolo-quinazolin-4(3H)-ones by Huisgen 1,3-dipolar cycloaddition with PI3K? isoform selective activity. Bioorg Med Chem Lett28:1005-1010 (2018) [PubMed] Article
Srinivas, M; Singh Pathania, A; Mahajan, P; Verma, PK; Chobe, SS; Malik, FA; Nargotra, A; Vishwakarma, RA; Sawant, SD Design and synthesis of 1,4-substituted 1H-1,2,3-triazolo-quinazolin-4(3H)-ones by Huisgen 1,3-dipolar cycloaddition with PI3K? isoform selective activity. Bioorg Med Chem Lett28:1005-1010 (2018) [PubMed] Article