 Found 50 hits with Last Name = 'srinivas' and Initial = 'm'
Found 50 hits with Last Name = 'srinivas' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067186
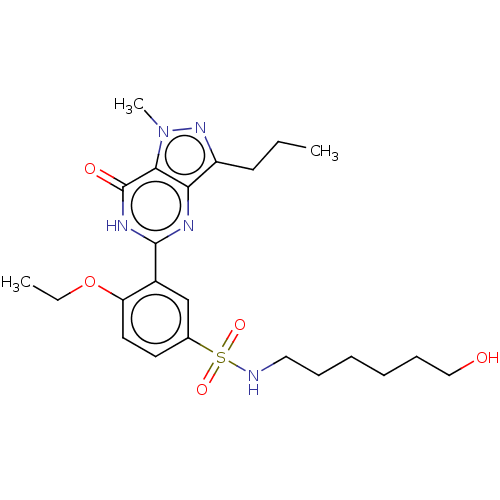
(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067195

(CHEMBL3401754)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H32N6O5S/c1-4-6-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(7-8-19(17)34-5-2)35(31,32)24-9-10-29-11-13-33-14-12-29/h7-8,15,24H,4-6,9-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067187

(CHEMBL3401746)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCO Show InChI InChI=1S/C22H31N5O5S/c1-4-9-17-19-20(27(3)26-17)22(29)25-21(24-19)16-14-15(10-11-18(16)32-5-2)33(30,31)23-12-7-6-8-13-28/h10-11,14,23,28H,4-9,12-13H2,1-3H3,(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE8A1 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067188
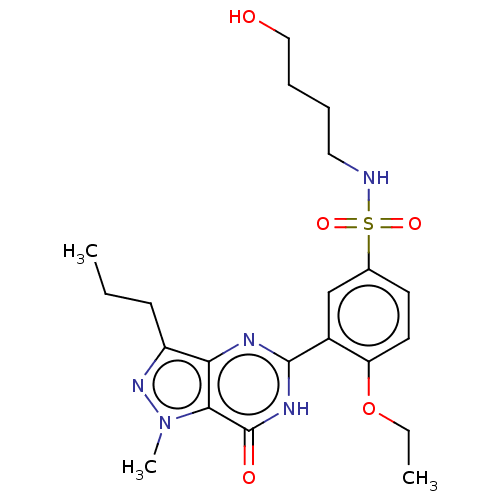
(CHEMBL3401747)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCO Show InChI InChI=1S/C21H29N5O5S/c1-4-8-16-18-19(26(3)25-16)21(28)24-20(23-18)15-13-14(9-10-17(15)31-5-2)32(29,30)22-11-6-7-12-27/h9-10,13,22,27H,4-8,11-12H2,1-3H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE7A1 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
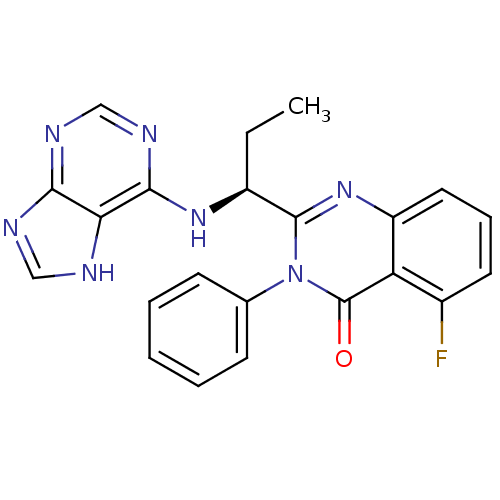
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-delta |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067194
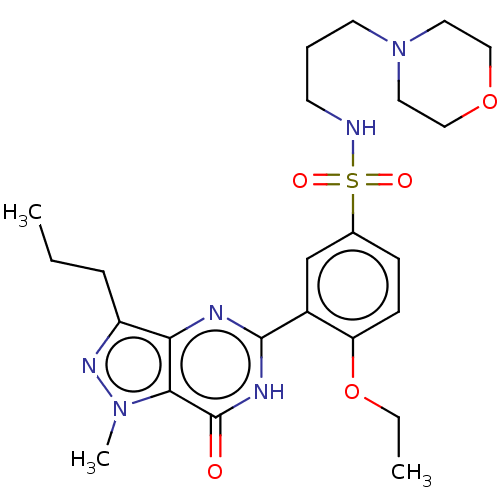
(CHEMBL3401753)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C24H34N6O5S/c1-4-7-19-21-22(29(3)28-19)24(31)27-23(26-21)18-16-17(8-9-20(18)35-5-2)36(32,33)25-10-6-11-30-12-14-34-15-13-30/h8-9,16,25H,4-7,10-15H2,1-3H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067198
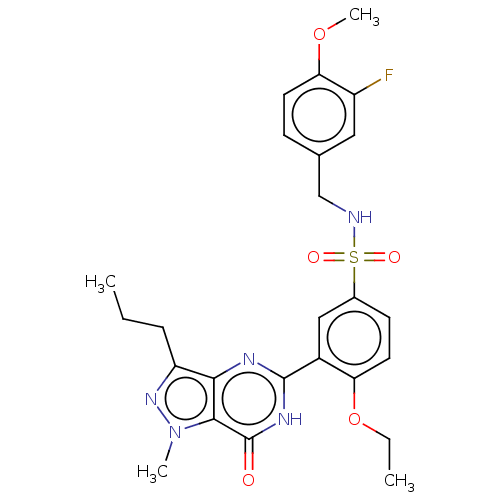
(CHEMBL3401757)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCc1ccc(OC)c(F)c1 Show InChI InChI=1S/C25H28FN5O5S/c1-5-7-19-22-23(31(3)30-19)25(32)29-24(28-22)17-13-16(9-11-20(17)36-6-2)37(33,34)27-14-15-8-10-21(35-4)18(26)12-15/h8-13,27H,5-7,14H2,1-4H3,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE11A4 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067189
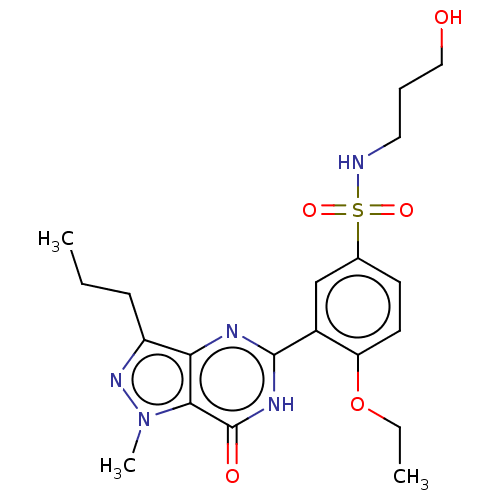
(CHEMBL3401748)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCO Show InChI InChI=1S/C20H27N5O5S/c1-4-7-15-17-18(25(3)24-15)20(27)23-19(22-17)14-12-13(8-9-16(14)30-5-2)31(28,29)21-10-6-11-26/h8-9,12,21,26H,4-7,10-11H2,1-3H3,(H,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE7A1 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067190

(CHEMBL3401749)Show SMILES CCCCCCCNS(=O)(=O)c1ccc(OCC)c(c1)-c1nc2c(CCC)nn(C)c2c(=O)[nH]1 Show InChI InChI=1S/C24H35N5O4S/c1-5-8-9-10-11-15-25-34(31,32)17-13-14-20(33-7-3)18(16-17)23-26-21-19(12-6-2)28-29(4)22(21)24(30)27-23/h13-14,16,25H,5-12,15H2,1-4H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE6C (unknown origin) |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE2A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067191
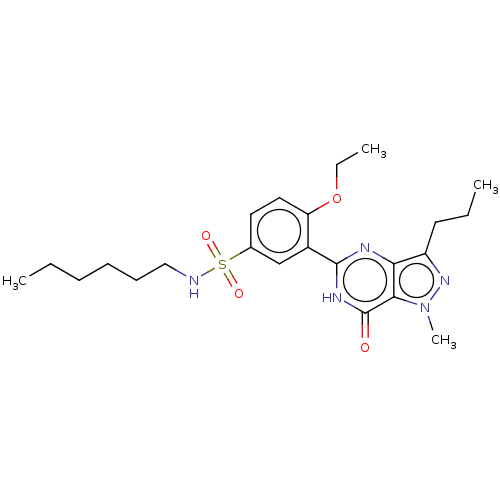
(CHEMBL3401750)Show SMILES CCCCCCNS(=O)(=O)c1ccc(OCC)c(c1)-c1nc2c(CCC)nn(C)c2c(=O)[nH]1 Show InChI InChI=1S/C23H33N5O4S/c1-5-8-9-10-14-24-33(30,31)16-12-13-19(32-7-3)17(15-16)22-25-20-18(11-6-2)27-28(4)21(20)23(29)26-22/h12-13,15,24H,5-11,14H2,1-4H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE6C (unknown origin) |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067197

(CHEMBL3401756)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C27H30N6O4S/c1-4-8-22-24-25(33(3)32-22)27(34)31-26(30-24)20-15-18(11-12-23(20)37-5-2)38(35,36)29-14-13-17-16-28-21-10-7-6-9-19(17)21/h6-7,9-12,15-16,28-29H,4-5,8,13-14H2,1-3H3,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE11A4 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067193

(CHEMBL3401752)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCN(C)C Show InChI InChI=1S/C22H32N6O4S/c1-6-9-17-19-20(28(5)26-17)22(29)25-21(24-19)16-14-15(10-11-18(16)32-7-2)33(30,31)23-12-8-13-27(3)4/h10-11,14,23H,6-9,12-13H2,1-5H3,(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 (unknown origin) |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067196

(CHEMBL3401755)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCN1CCCCC1 Show InChI InChI=1S/C24H34N6O4S/c1-4-9-19-21-22(29(3)28-19)24(31)27-23(26-21)18-16-17(10-11-20(18)34-5-2)35(32,33)25-12-15-30-13-7-6-8-14-30/h10-11,16,25H,4-9,12-15H2,1-3H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE2A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067192
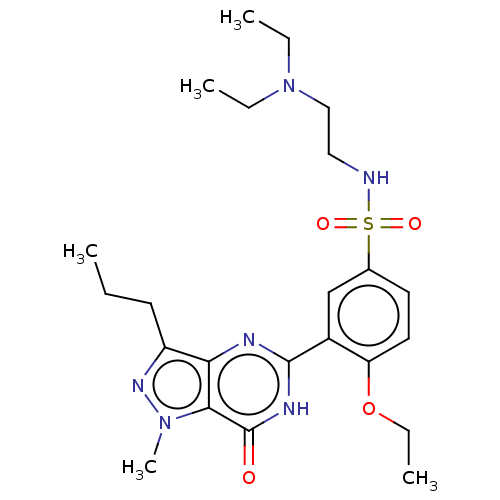
(CHEMBL3401751)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCN(CC)CC Show InChI InChI=1S/C23H34N6O4S/c1-6-10-18-20-21(28(5)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-9-4)34(31,32)24-13-14-29(7-2)8-3/h11-12,15,24H,6-10,13-14H2,1-5H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 (unknown origin) |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE1A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323726
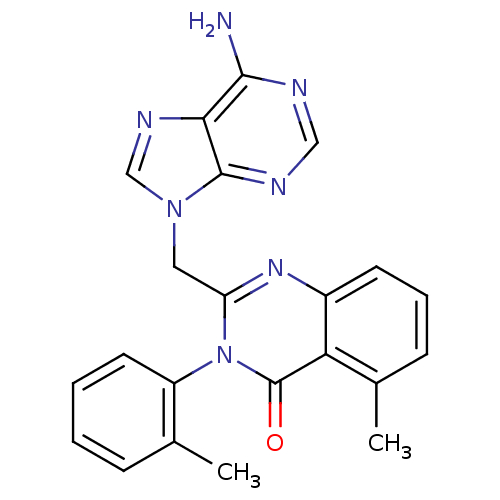
(2-((6-amino-9H-purin-9-yl)methyl)-5-methyl-3-o-tol...)Show SMILES Cc1ccccc1-n1c(Cn2cnc3c(N)ncnc23)nc2cccc(C)c2c1=O |(5.33,-1.02,;5.32,.52,;6.65,1.29,;6.64,2.84,;5.29,3.6,;3.97,2.82,;3.98,1.28,;2.65,.51,;2.67,-1.03,;4.01,-1.8,;4.01,-3.34,;2.77,-4.26,;3.24,-5.72,;4.78,-5.71,;5.82,-6.85,;5.34,-8.31,;7.31,-6.53,;7.78,-5.06,;6.75,-3.93,;5.26,-4.25,;1.33,-1.82,;-.01,-1.04,;-1.35,-1.83,;-2.67,-1.05,;-2.67,.49,;-1.35,1.26,;-1.35,2.8,;-.01,.5,;1.31,1.27,;1.31,2.81,)| Show InChI InChI=1S/C22H19N7O/c1-13-6-3-4-9-16(13)29-17(27-15-8-5-7-14(2)18(15)22(29)30)10-28-12-26-19-20(23)24-11-25-21(19)28/h3-9,11-12H,10H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) expressed in baculovirus infected Sf9 insect cells after 10 mins in presence of [gamma-32P]ATP by radioliga... |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323726

(2-((6-amino-9H-purin-9-yl)methyl)-5-methyl-3-o-tol...)Show SMILES Cc1ccccc1-n1c(Cn2cnc3c(N)ncnc23)nc2cccc(C)c2c1=O |(5.33,-1.02,;5.32,.52,;6.65,1.29,;6.64,2.84,;5.29,3.6,;3.97,2.82,;3.98,1.28,;2.65,.51,;2.67,-1.03,;4.01,-1.8,;4.01,-3.34,;2.77,-4.26,;3.24,-5.72,;4.78,-5.71,;5.82,-6.85,;5.34,-8.31,;7.31,-6.53,;7.78,-5.06,;6.75,-3.93,;5.26,-4.25,;1.33,-1.82,;-.01,-1.04,;-1.35,-1.83,;-2.67,-1.05,;-2.67,.49,;-1.35,1.26,;-1.35,2.8,;-.01,.5,;1.31,1.27,;1.31,2.81,)| Show InChI InChI=1S/C22H19N7O/c1-13-6-3-4-9-16(13)29-17(27-15-8-5-7-14(2)18(15)22(29)30)10-28-12-26-19-20(23)24-11-25-21(19)28/h3-9,11-12H,10H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50460339
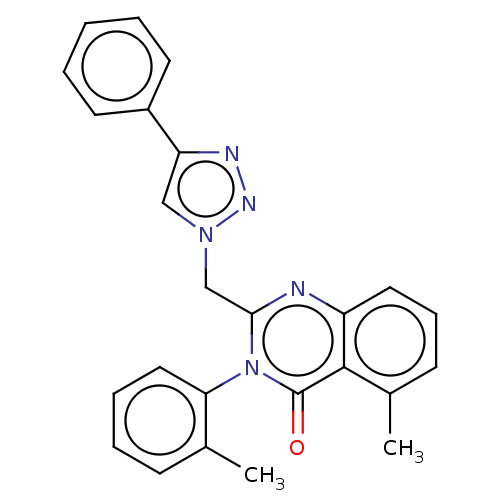
(CHEMBL4229035)Show SMILES Cc1ccccc1-n1c(Cn2cc(nn2)-c2ccccc2)nc2cccc(C)c2c1=O |(24.56,-20.94,;25.89,-20.17,;25.89,-18.63,;27.22,-17.86,;28.56,-18.63,;28.56,-20.17,;27.22,-20.94,;27.22,-22.48,;25.89,-23.25,;24.56,-22.48,;23.22,-23.25,;21.82,-22.62,;20.79,-23.77,;21.56,-25.1,;23.06,-24.78,;19.25,-23.74,;18.45,-25.05,;16.91,-25.02,;16.17,-23.68,;16.96,-22.36,;18.5,-22.39,;25.89,-24.79,;27.22,-25.56,;27.22,-27.1,;28.56,-27.87,;29.89,-27.1,;29.89,-25.56,;31.23,-24.79,;28.56,-24.79,;28.56,-23.25,;29.89,-22.48,)| Show InChI InChI=1S/C25H21N5O/c1-17-9-6-7-14-22(17)30-23(26-20-13-8-10-18(2)24(20)25(30)31)16-29-15-21(27-28-29)19-11-4-3-5-12-19/h3-15H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE9A2 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50460340

(CHEMBL4228200)Show SMILES COc1ccc(-c2cn(Cc3nc4cccc(C)c4c(=O)n3-c3ccccc3C)nn2)c(C)c1 |(27.62,-21.79,;28.98,-21.07,;30.29,-21.88,;31.65,-21.16,;32.95,-21.98,;32.89,-23.52,;34.2,-24.34,;35.44,-23.43,;36.69,-24.32,;38.15,-23.84,;39.47,-24.63,;39.44,-26.17,;40.76,-26.97,;40.73,-28.51,;42.05,-29.3,;43.4,-28.56,;43.43,-27.02,;44.77,-26.28,;42.11,-26.22,;42.14,-24.69,;43.49,-23.94,;40.82,-23.89,;40.8,-22.35,;42.13,-21.56,;42.11,-20.02,;40.76,-19.27,;39.44,-20.06,;39.46,-21.6,;38.13,-22.38,;36.23,-25.79,;34.69,-25.8,;31.53,-24.24,;31.48,-25.78,;30.23,-23.42,)| Show InChI InChI=1S/C27H25N5O2/c1-17-8-5-6-11-24(17)32-25(28-22-10-7-9-18(2)26(22)27(32)33)16-31-15-23(29-30-31)21-13-12-20(34-4)14-19(21)3/h5-15H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE9A2 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50460339

(CHEMBL4229035)Show SMILES Cc1ccccc1-n1c(Cn2cc(nn2)-c2ccccc2)nc2cccc(C)c2c1=O |(24.56,-20.94,;25.89,-20.17,;25.89,-18.63,;27.22,-17.86,;28.56,-18.63,;28.56,-20.17,;27.22,-20.94,;27.22,-22.48,;25.89,-23.25,;24.56,-22.48,;23.22,-23.25,;21.82,-22.62,;20.79,-23.77,;21.56,-25.1,;23.06,-24.78,;19.25,-23.74,;18.45,-25.05,;16.91,-25.02,;16.17,-23.68,;16.96,-22.36,;18.5,-22.39,;25.89,-24.79,;27.22,-25.56,;27.22,-27.1,;28.56,-27.87,;29.89,-27.1,;29.89,-25.56,;31.23,-24.79,;28.56,-24.79,;28.56,-23.25,;29.89,-22.48,)| Show InChI InChI=1S/C25H21N5O/c1-17-9-6-7-14-22(17)30-23(26-20-13-8-10-18(2)24(20)25(30)31)16-29-15-21(27-28-29)19-11-4-3-5-12-19/h3-15H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50460340

(CHEMBL4228200)Show SMILES COc1ccc(-c2cn(Cc3nc4cccc(C)c4c(=O)n3-c3ccccc3C)nn2)c(C)c1 |(27.62,-21.79,;28.98,-21.07,;30.29,-21.88,;31.65,-21.16,;32.95,-21.98,;32.89,-23.52,;34.2,-24.34,;35.44,-23.43,;36.69,-24.32,;38.15,-23.84,;39.47,-24.63,;39.44,-26.17,;40.76,-26.97,;40.73,-28.51,;42.05,-29.3,;43.4,-28.56,;43.43,-27.02,;44.77,-26.28,;42.11,-26.22,;42.14,-24.69,;43.49,-23.94,;40.82,-23.89,;40.8,-22.35,;42.13,-21.56,;42.11,-20.02,;40.76,-19.27,;39.44,-20.06,;39.46,-21.6,;38.13,-22.38,;36.23,-25.79,;34.69,-25.8,;31.53,-24.24,;31.48,-25.78,;30.23,-23.42,)| Show InChI InChI=1S/C27H25N5O2/c1-17-8-5-6-11-24(17)32-25(28-22-10-7-9-18(2)26(22)27(32)33)16-31-15-23(29-30-31)21-13-12-20(34-4)14-19(21)3/h5-15H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human PDE8A1 |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50067186

(CHEMBL3401745)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)NCCCCCCO Show InChI InChI=1S/C23H33N5O5S/c1-4-10-18-20-21(28(3)27-18)23(30)26-22(25-20)17-15-16(11-12-19(17)33-5-2)34(31,32)24-13-8-6-7-9-14-29/h11-12,15,24,29H,4-10,13-14H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE1A1 (unknown origin) using fluorescently labeled cAMP substrate by fluorescence polarization assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50323726

(2-((6-amino-9H-purin-9-yl)methyl)-5-methyl-3-o-tol...)Show SMILES Cc1ccccc1-n1c(Cn2cnc3c(N)ncnc23)nc2cccc(C)c2c1=O |(5.33,-1.02,;5.32,.52,;6.65,1.29,;6.64,2.84,;5.29,3.6,;3.97,2.82,;3.98,1.28,;2.65,.51,;2.67,-1.03,;4.01,-1.8,;4.01,-3.34,;2.77,-4.26,;3.24,-5.72,;4.78,-5.71,;5.82,-6.85,;5.34,-8.31,;7.31,-6.53,;7.78,-5.06,;6.75,-3.93,;5.26,-4.25,;1.33,-1.82,;-.01,-1.04,;-1.35,-1.83,;-2.67,-1.05,;-2.67,.49,;-1.35,1.26,;-1.35,2.8,;-.01,.5,;1.31,1.27,;1.31,2.81,)| Show InChI InChI=1S/C22H19N7O/c1-13-6-3-4-9-16(13)29-17(27-15-8-5-7-14(2)18(15)22(29)30)10-28-12-26-19-20(23)24-11-25-21(19)28/h3-9,11-12H,10H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 expressed in baculovirus in sf9 cells by PDE Glo phosphodiesterase assay |
Bioorg Med Chem 23: 2121-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.005
BindingDB Entry DOI: 10.7270/Q28917JN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50323726

(2-((6-amino-9H-purin-9-yl)methyl)-5-methyl-3-o-tol...)Show SMILES Cc1ccccc1-n1c(Cn2cnc3c(N)ncnc23)nc2cccc(C)c2c1=O |(5.33,-1.02,;5.32,.52,;6.65,1.29,;6.64,2.84,;5.29,3.6,;3.97,2.82,;3.98,1.28,;2.65,.51,;2.67,-1.03,;4.01,-1.8,;4.01,-3.34,;2.77,-4.26,;3.24,-5.72,;4.78,-5.71,;5.82,-6.85,;5.34,-8.31,;7.31,-6.53,;7.78,-5.06,;6.75,-3.93,;5.26,-4.25,;1.33,-1.82,;-.01,-1.04,;-1.35,-1.83,;-2.67,-1.05,;-2.67,.49,;-1.35,1.26,;-1.35,2.8,;-.01,.5,;1.31,1.27,;1.31,2.81,)| Show InChI InChI=1S/C22H19N7O/c1-13-6-3-4-9-16(13)29-17(27-15-8-5-7-14(2)18(15)22(29)30)10-28-12-26-19-20(23)24-11-25-21(19)28/h3-9,11-12H,10H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50460339

(CHEMBL4229035)Show SMILES Cc1ccccc1-n1c(Cn2cc(nn2)-c2ccccc2)nc2cccc(C)c2c1=O |(24.56,-20.94,;25.89,-20.17,;25.89,-18.63,;27.22,-17.86,;28.56,-18.63,;28.56,-20.17,;27.22,-20.94,;27.22,-22.48,;25.89,-23.25,;24.56,-22.48,;23.22,-23.25,;21.82,-22.62,;20.79,-23.77,;21.56,-25.1,;23.06,-24.78,;19.25,-23.74,;18.45,-25.05,;16.91,-25.02,;16.17,-23.68,;16.96,-22.36,;18.5,-22.39,;25.89,-24.79,;27.22,-25.56,;27.22,-27.1,;28.56,-27.87,;29.89,-27.1,;29.89,-25.56,;31.23,-24.79,;28.56,-24.79,;28.56,-23.25,;29.89,-22.48,)| Show InChI InChI=1S/C25H21N5O/c1-17-9-6-7-14-22(17)30-23(26-20-13-8-10-18(2)24(20)25(30)31)16-29-15-21(27-28-29)19-11-4-3-5-12-19/h3-15H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50323726

(2-((6-amino-9H-purin-9-yl)methyl)-5-methyl-3-o-tol...)Show SMILES Cc1ccccc1-n1c(Cn2cnc3c(N)ncnc23)nc2cccc(C)c2c1=O |(5.33,-1.02,;5.32,.52,;6.65,1.29,;6.64,2.84,;5.29,3.6,;3.97,2.82,;3.98,1.28,;2.65,.51,;2.67,-1.03,;4.01,-1.8,;4.01,-3.34,;2.77,-4.26,;3.24,-5.72,;4.78,-5.71,;5.82,-6.85,;5.34,-8.31,;7.31,-6.53,;7.78,-5.06,;6.75,-3.93,;5.26,-4.25,;1.33,-1.82,;-.01,-1.04,;-1.35,-1.83,;-2.67,-1.05,;-2.67,.49,;-1.35,1.26,;-1.35,2.8,;-.01,.5,;1.31,1.27,;1.31,2.81,)| Show InChI InChI=1S/C22H19N7O/c1-13-6-3-4-9-16(13)29-17(27-15-8-5-7-14(2)18(15)22(29)30)10-28-12-26-19-20(23)24-11-25-21(19)28/h3-9,11-12H,10H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50460340

(CHEMBL4228200)Show SMILES COc1ccc(-c2cn(Cc3nc4cccc(C)c4c(=O)n3-c3ccccc3C)nn2)c(C)c1 |(27.62,-21.79,;28.98,-21.07,;30.29,-21.88,;31.65,-21.16,;32.95,-21.98,;32.89,-23.52,;34.2,-24.34,;35.44,-23.43,;36.69,-24.32,;38.15,-23.84,;39.47,-24.63,;39.44,-26.17,;40.76,-26.97,;40.73,-28.51,;42.05,-29.3,;43.4,-28.56,;43.43,-27.02,;44.77,-26.28,;42.11,-26.22,;42.14,-24.69,;43.49,-23.94,;40.82,-23.89,;40.8,-22.35,;42.13,-21.56,;42.11,-20.02,;40.76,-19.27,;39.44,-20.06,;39.46,-21.6,;38.13,-22.38,;36.23,-25.79,;34.69,-25.8,;31.53,-24.24,;31.48,-25.78,;30.23,-23.42,)| Show InChI InChI=1S/C27H25N5O2/c1-17-8-5-6-11-24(17)32-25(28-22-10-7-9-18(2)26(22)27(32)33)16-31-15-23(29-30-31)21-13-12-20(34-4)14-19(21)3/h5-15H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50460340

(CHEMBL4228200)Show SMILES COc1ccc(-c2cn(Cc3nc4cccc(C)c4c(=O)n3-c3ccccc3C)nn2)c(C)c1 |(27.62,-21.79,;28.98,-21.07,;30.29,-21.88,;31.65,-21.16,;32.95,-21.98,;32.89,-23.52,;34.2,-24.34,;35.44,-23.43,;36.69,-24.32,;38.15,-23.84,;39.47,-24.63,;39.44,-26.17,;40.76,-26.97,;40.73,-28.51,;42.05,-29.3,;43.4,-28.56,;43.43,-27.02,;44.77,-26.28,;42.11,-26.22,;42.14,-24.69,;43.49,-23.94,;40.82,-23.89,;40.8,-22.35,;42.13,-21.56,;42.11,-20.02,;40.76,-19.27,;39.44,-20.06,;39.46,-21.6,;38.13,-22.38,;36.23,-25.79,;34.69,-25.8,;31.53,-24.24,;31.48,-25.78,;30.23,-23.42,)| Show InChI InChI=1S/C27H25N5O2/c1-17-8-5-6-11-24(17)32-25(28-22-10-7-9-18(2)26(22)27(32)33)16-31-15-23(29-30-31)21-13-12-20(34-4)14-19(21)3/h5-15H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50460339

(CHEMBL4229035)Show SMILES Cc1ccccc1-n1c(Cn2cc(nn2)-c2ccccc2)nc2cccc(C)c2c1=O |(24.56,-20.94,;25.89,-20.17,;25.89,-18.63,;27.22,-17.86,;28.56,-18.63,;28.56,-20.17,;27.22,-20.94,;27.22,-22.48,;25.89,-23.25,;24.56,-22.48,;23.22,-23.25,;21.82,-22.62,;20.79,-23.77,;21.56,-25.1,;23.06,-24.78,;19.25,-23.74,;18.45,-25.05,;16.91,-25.02,;16.17,-23.68,;16.96,-22.36,;18.5,-22.39,;25.89,-24.79,;27.22,-25.56,;27.22,-27.1,;28.56,-27.87,;29.89,-27.1,;29.89,-25.56,;31.23,-24.79,;28.56,-24.79,;28.56,-23.25,;29.89,-22.48,)| Show InChI InChI=1S/C25H21N5O/c1-17-9-6-7-14-22(17)30-23(26-20-13-8-10-18(2)24(20)25(30)31)16-29-15-21(27-28-29)19-11-4-3-5-12-19/h3-15H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Academy of Scientific & Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) by cell free biochemical assay |
Bioorg Med Chem Lett 28: 1005-1010 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.032
BindingDB Entry DOI: 10.7270/Q29S1TN5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data