| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Oxysterols receptor LXR-beta |
|---|
| Ligand | BDBM50461063 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1770683 (CHEMBL4222795) |
|---|
| Kd | >10000±n/a nM |
|---|
| Citation |  Ouvry, G; Bihl, F; Bouix-Peter, C; Christin, O; Defoin-Platel, C; Deret, S; Feret, C; Froude, D; Hacini-Rachinel, F; Harris, CS; Hervouet, C; Lafitte, G; Luzy, AP; Musicki, B; Orfila, D; Parnet, V; Pascau, C; Pascau, J; Pierre, R; Raffin, C; Rossio, P; Spiesse, D; Taquet, N; Thoreau, E; Vatinel, R; Vial, E; Hennequin, LF Sulfoximines as potent ROR? inverse agonists. Bioorg Med Chem Lett28:1269-1273 (2018) [PubMed] Article Ouvry, G; Bihl, F; Bouix-Peter, C; Christin, O; Defoin-Platel, C; Deret, S; Feret, C; Froude, D; Hacini-Rachinel, F; Harris, CS; Hervouet, C; Lafitte, G; Luzy, AP; Musicki, B; Orfila, D; Parnet, V; Pascau, C; Pascau, J; Pierre, R; Raffin, C; Rossio, P; Spiesse, D; Taquet, N; Thoreau, E; Vatinel, R; Vial, E; Hennequin, LF Sulfoximines as potent ROR? inverse agonists. Bioorg Med Chem Lett28:1269-1273 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Oxysterols receptor LXR-beta |
|---|
| Name: | Oxysterols receptor LXR-beta |
|---|
| Synonyms: | LXRB | Liver X receptor beta (NR1H2) | Liver X, LXR beta | NER | NR1H2 | NR1H2_HUMAN | Nuclear receptor NER | UNR | Ubiquitously-expressed nuclear receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 50978.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P55055 |
|---|
| Residue: | 460 |
|---|
| Sequence: | MSSPTTSSLDTPLPGNGPPQPGAPSSSPTVKEEGPEPWPGGPDPDVPGTDEASSACSTDW
VIPDPEEEPERKRKKGPAPKMLGHELCRVCGDKASGFHYNVLSCEGCKGFFRRSVVRGGA
RRYACRGGGTCQMDAFMRRKCQQCRLRKCKEAGMREQCVLSEEQIRKKKIRKQQQESQSQ
SQSPVGPQGSSSSASGPGASPGGSEAGSQGSGEGEGVQLTAAQELMIQQLVAAQLQCNKR
SFSDQPKVTPWPLGADPQSRDARQQRFAHFTELAIISVQEIVDFAKQVPGFLQLGREDQI
ALLKASTIEIMLLETARRYNHETECITFLKDFTYSKDDFHRAGLQVEFINPIFEFSRAMR
RLGLDDAEYALLIAINIFSADRPNVQEPGRVEALQQPYVEALLSYTRIKRPQDQLRFPRM
LMKLVSLRTLSSVHSEQVFALRLQDKKLPPLLSEIWDVHE
|
|
|
|---|
| BDBM50461063 |
|---|
| n/a |
|---|
| Name | BDBM50461063 |
|---|
| Synonyms: | CHEMBL4226803 | US10457637, Compound 8 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H36N2O5S2 |
|---|
| Mol. Mass. | 508.694 |
|---|
| SMILES | CCc1ccc(cc1)N(CC(C)C)S(=O)(=O)c1ccc(OCC2CCOCC2)c(c1)[S@@](C)(=N)=O |r| |
|---|
| Structure | 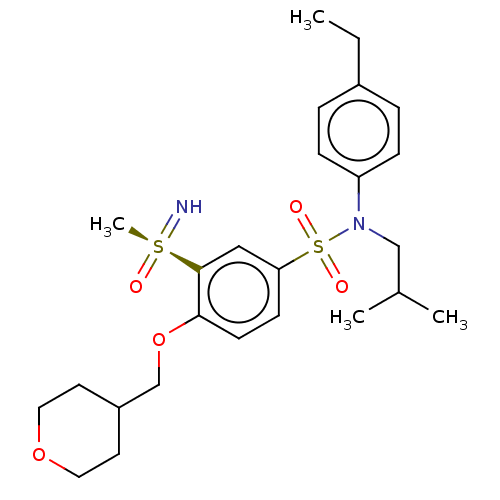
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ouvry, G; Bihl, F; Bouix-Peter, C; Christin, O; Defoin-Platel, C; Deret, S; Feret, C; Froude, D; Hacini-Rachinel, F; Harris, CS; Hervouet, C; Lafitte, G; Luzy, AP; Musicki, B; Orfila, D; Parnet, V; Pascau, C; Pascau, J; Pierre, R; Raffin, C; Rossio, P; Spiesse, D; Taquet, N; Thoreau, E; Vatinel, R; Vial, E; Hennequin, LF Sulfoximines as potent ROR? inverse agonists. Bioorg Med Chem Lett28:1269-1273 (2018) [PubMed] Article
Ouvry, G; Bihl, F; Bouix-Peter, C; Christin, O; Defoin-Platel, C; Deret, S; Feret, C; Froude, D; Hacini-Rachinel, F; Harris, CS; Hervouet, C; Lafitte, G; Luzy, AP; Musicki, B; Orfila, D; Parnet, V; Pascau, C; Pascau, J; Pierre, R; Raffin, C; Rossio, P; Spiesse, D; Taquet, N; Thoreau, E; Vatinel, R; Vial, E; Hennequin, LF Sulfoximines as potent ROR? inverse agonists. Bioorg Med Chem Lett28:1269-1273 (2018) [PubMed] Article