| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase BTK |
|---|
| Ligand | BDBM165253 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1778704 (CHEMBL4235696) |
|---|
| IC50 | 12±n/a nM |
|---|
| Citation |  Liu, Q; Batt, DG; Chaudhry, C; Lippy, JS; Pattoli, MA; Surti, N; Xu, S; Carter, PH; Burke, JR; Tino, JA Conversion of carbazole carboxamide based reversible inhibitors of Bruton's tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series. Bioorg Med Chem Lett28:3080-3084 (2018) [PubMed] Article Liu, Q; Batt, DG; Chaudhry, C; Lippy, JS; Pattoli, MA; Surti, N; Xu, S; Carter, PH; Burke, JR; Tino, JA Conversion of carbazole carboxamide based reversible inhibitors of Bruton's tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series. Bioorg Med Chem Lett28:3080-3084 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase BTK |
|---|
| Name: | Tyrosine-protein kinase BTK |
|---|
| Synonyms: | AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 76289.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q06187 |
|---|
| Residue: | 659 |
|---|
| Sequence: | MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEK
ITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEEL
RKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSS
HRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDE
YFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGK
EGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELIN
YHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGK
WRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANG
CLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDF
GLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYER
FTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
|
|
|
|---|
| BDBM165253 |
|---|
| n/a |
|---|
| Name | BDBM165253 |
|---|
| Synonyms: | US10604504, Example 49 | US11623921, Example 49 | US9688629, 49 | US9802915, Example 49 | US9920031, Example 49 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H22N4O2 |
|---|
| Mol. Mass. | 422.4785 |
|---|
| SMILES | Cc1[nH]c2c(ccc(-c3cccc(c3C)-n3cnc4ccccc4c3=O)c2c1C)C(N)=O |
|---|
| Structure | 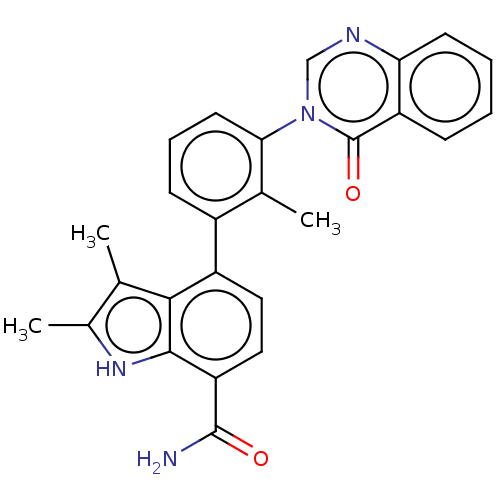
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, Q; Batt, DG; Chaudhry, C; Lippy, JS; Pattoli, MA; Surti, N; Xu, S; Carter, PH; Burke, JR; Tino, JA Conversion of carbazole carboxamide based reversible inhibitors of Bruton's tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series. Bioorg Med Chem Lett28:3080-3084 (2018) [PubMed] Article
Liu, Q; Batt, DG; Chaudhry, C; Lippy, JS; Pattoli, MA; Surti, N; Xu, S; Carter, PH; Burke, JR; Tino, JA Conversion of carbazole carboxamide based reversible inhibitors of Bruton's tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series. Bioorg Med Chem Lett28:3080-3084 (2018) [PubMed] Article