

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Receptor-type tyrosine-protein kinase FLT3 | ||
| Ligand | BDBM50469364 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1804043 (CHEMBL4276335) | ||
| IC50 | 3.4±n/a nM | ||
| Citation |  Zhao, J; Zhang, D; Zhang, W; Stashko, MA; DeRyckere, D; Vasileiadi, E; Parker, RE; Hunter, D; Liu, Q; Zhang, Y; Norris-Drouin, J; Li, B; Drewry, DH; Kireev, D; Graham, DK; Earp, HS; Frye, SV; Wang, X Highly Selective MERTK Inhibitors Achieved by a Single Methyl Group. J Med Chem61:10242-10254 (2018) [PubMed] Article Zhao, J; Zhang, D; Zhang, W; Stashko, MA; DeRyckere, D; Vasileiadi, E; Parker, RE; Hunter, D; Liu, Q; Zhang, Y; Norris-Drouin, J; Li, B; Drewry, DH; Kireev, D; Graham, DK; Earp, HS; Frye, SV; Wang, X Highly Selective MERTK Inhibitors Achieved by a Single Methyl Group. J Med Chem61:10242-10254 (2018) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Receptor-type tyrosine-protein kinase FLT3 | |||
| Name: | Receptor-type tyrosine-protein kinase FLT3 | ||
| Synonyms: | CD135 | CD_antigen: CD135 | FL cytokine receptor | FLK-2 | FLK2 | FLT-3 | FLT3 | FLT3_HUMAN | Fetal liver kinase-2 | Fms-like tyrosine kinase 3 | Fms-like tyrosine kinase 3 (Flt-3) | Fms-related tyrosine kinase 3 | STK-1 | STK1 | Stem cell tyrosine kinase 1 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 112888.62 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P36888 | ||
| Residue: | 993 | ||
| Sequence: |
| ||
| BDBM50469364 | |||
| n/a | |||
| Name | BDBM50469364 | ||
| Synonyms: | CHEMBL4291006 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C27H38N6O | ||
| Mol. Mass. | 462.6302 | ||
| SMILES | CCCCNc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(NC2CCNCC2)cc1 |r,wU:12.11,wD:15.15,(20.69,-20.42,;20.68,-21.96,;22.01,-22.74,;23.34,-21.97,;24.67,-22.74,;26.01,-21.97,;26.01,-20.43,;27.34,-19.66,;28.67,-20.42,;30.15,-19.94,;31.06,-21.2,;30.15,-22.45,;30.89,-23.79,;32.43,-23.82,;33.17,-25.17,;32.37,-26.49,;33.12,-27.84,;30.84,-26.45,;30.1,-25.11,;28.67,-21.97,;27.34,-22.74,;30.86,-18.58,;32.4,-18.52,;33.11,-17.16,;32.29,-15.85,;32.99,-14.49,;34.53,-14.42,;35.35,-15.72,;36.88,-15.66,;37.6,-14.3,;36.78,-12.99,;35.23,-13.05,;30.74,-15.92,;30.03,-17.29,)| | ||
| Structure | 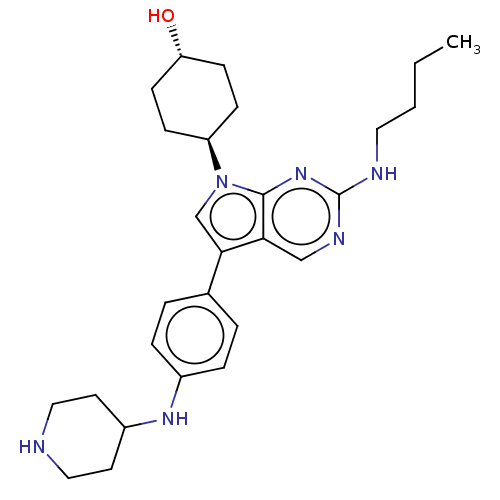
| ||