

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Reverse transcriptase | ||
| Ligand | BDBM50485631 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_842481 (CHEMBL2092206) | ||
| IC50 | 4400±n/a nM | ||
| Citation |  Li, X; Zhan, P; Liu, H; Li, D; Wang, L; Chen, X; Liu, H; Pannecouque, C; Balzarini, J; De Clercq, E; Liu, X Arylazolyl(azinyl)thioacetanilides. Part 10: design, synthesis and biological evaluation of novel substituted imidazopyridinylthioacetanilides as potent HIV-1 inhibitors. Bioorg Med Chem20:5527-36 (2012) [PubMed] Article Li, X; Zhan, P; Liu, H; Li, D; Wang, L; Chen, X; Liu, H; Pannecouque, C; Balzarini, J; De Clercq, E; Liu, X Arylazolyl(azinyl)thioacetanilides. Part 10: design, synthesis and biological evaluation of novel substituted imidazopyridinylthioacetanilides as potent HIV-1 inhibitors. Bioorg Med Chem20:5527-36 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Reverse transcriptase | |||
| Name: | Reverse transcriptase | ||
| Synonyms: | n/a | ||
| Type: | Protein | ||
| Mol. Mass.: | 29598.37 | ||
| Organism: | Human immunodeficiency virus 1 | ||
| Description: | Q9WKE8 | ||
| Residue: | 254 | ||
| Sequence: |
| ||
| BDBM50485631 | |||
| n/a | |||
| Name | BDBM50485631 | ||
| Synonyms: | CHEMBL2088335 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H22BrN5O3S2 | ||
| Mol. Mass. | 560.486 | ||
| SMILES | Cc1cc(C)c(c(C)c1)-n1c(SCC(=O)Nc2ccc(cc2Br)S(N)(=O)=O)nc2cnccc12 |(-.67,-53.17,;-1.42,-51.82,;-2.96,-51.79,;-3.7,-50.45,;-5.24,-50.42,;-2.9,-49.13,;-1.37,-49.15,;-.58,-47.83,;-.62,-50.49,;-3.65,-47.78,;-2.75,-46.53,;-1.21,-46.52,;-.45,-45.18,;1.09,-45.17,;1.87,-46.5,;1.85,-43.84,;3.39,-43.83,;4.17,-45.16,;5.71,-45.15,;6.47,-43.81,;5.68,-42.48,;4.15,-42.5,;3.36,-41.17,;8,-43.8,;8.79,-45.13,;8,-42.26,;9.33,-43.02,;-3.67,-45.29,;-5.13,-45.77,;-6.46,-45.01,;-7.79,-45.78,;-7.79,-47.32,;-6.46,-48.1,;-5.12,-47.32,)| | ||
| Structure | 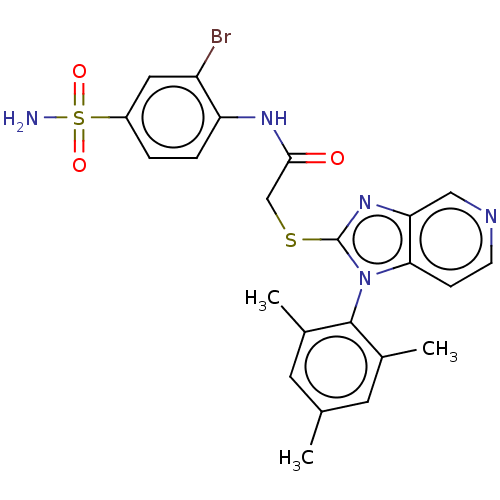
| ||