| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50499445 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1540696 (CHEMBL3744963) |
|---|
| IC50 | 25500±n/a nM |
|---|
| Citation |  Nirogi, R; Mohammed, AR; Shinde, AK; Bogaraju, N; Gagginapalli, SR; Ravella, SR; Kota, L; Bhyrapuneni, G; Muddana, NR; Benade, V; Palacharla, RC; Jayarajan, P; Subramanian, R; Goyal, VK Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer's disease. Eur J Med Chem103:289-301 (2015) [PubMed] Article Nirogi, R; Mohammed, AR; Shinde, AK; Bogaraju, N; Gagginapalli, SR; Ravella, SR; Kota, L; Bhyrapuneni, G; Muddana, NR; Benade, V; Palacharla, RC; Jayarajan, P; Subramanian, R; Goyal, VK Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer's disease. Eur J Med Chem103:289-301 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50499445 |
|---|
| n/a |
|---|
| Name | BDBM50499445 |
|---|
| Synonyms: | CHEMBL3741608 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H34N4O2 |
|---|
| Mol. Mass. | 398.5417 |
|---|
| SMILES | CC(C)c1nc(C(=O)NCC2CCN(CC3CCOCC3)CC2)c2ccccn12 |
|---|
| Structure | 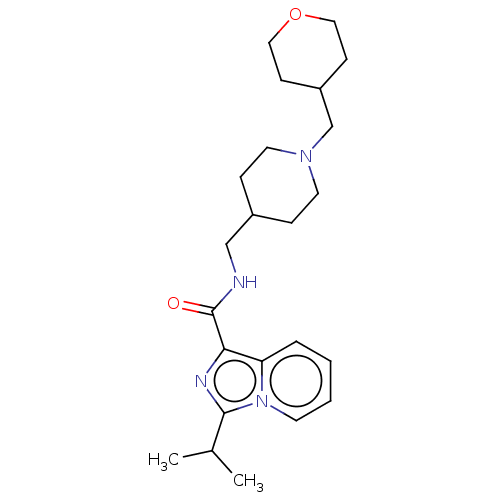
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nirogi, R; Mohammed, AR; Shinde, AK; Bogaraju, N; Gagginapalli, SR; Ravella, SR; Kota, L; Bhyrapuneni, G; Muddana, NR; Benade, V; Palacharla, RC; Jayarajan, P; Subramanian, R; Goyal, VK Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer's disease. Eur J Med Chem103:289-301 (2015) [PubMed] Article
Nirogi, R; Mohammed, AR; Shinde, AK; Bogaraju, N; Gagginapalli, SR; Ravella, SR; Kota, L; Bhyrapuneni, G; Muddana, NR; Benade, V; Palacharla, RC; Jayarajan, P; Subramanian, R; Goyal, VK Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer's disease. Eur J Med Chem103:289-301 (2015) [PubMed] Article