 Found 687 hits with Last Name = 'shinde' and Initial = 'ak'
Found 687 hits with Last Name = 'shinde' and Initial = 'ak' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM221048
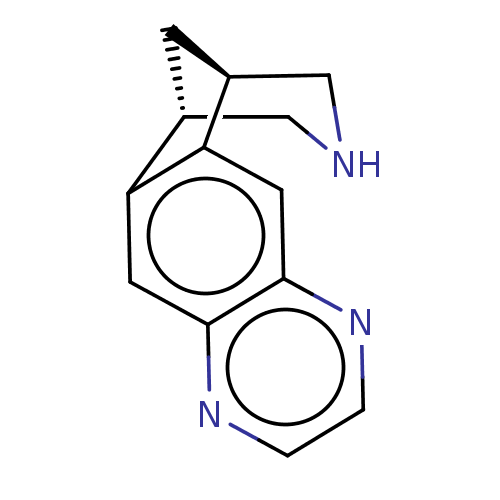
(US9284322, varenicline | US9303017, Varenicline)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2/t8-,9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538045
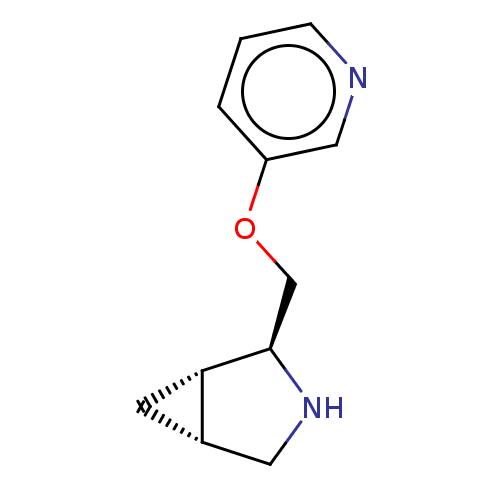
(CHEMBL4636163)Show SMILES Cl.[H][C@@]12C[C@]1([H])[C@@H](COc1cccnc1)NC2 |r| Show InChI InChI=1S/C11H14N2O.ClH/c1-2-9(6-12-3-1)14-7-11-10-4-8(10)5-13-11;/h1-3,6,8,10-11,13H,4-5,7H2;1H/t8-,10-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538008
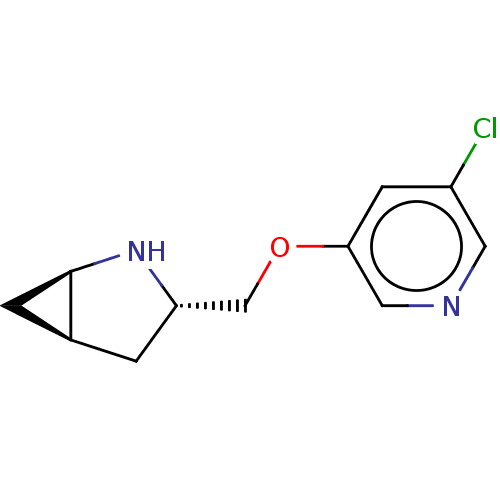
(CHEMBL4638732)Show SMILES Cl.[H][C@]12C[C@@]1([H])N[C@H](COc1cncc(Cl)c1)C2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-8-3-10(5-13-4-8)15-6-9-1-7-2-11(7)14-9;/h3-5,7,9,11,14H,1-2,6H2;1H/t7-,9-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538046
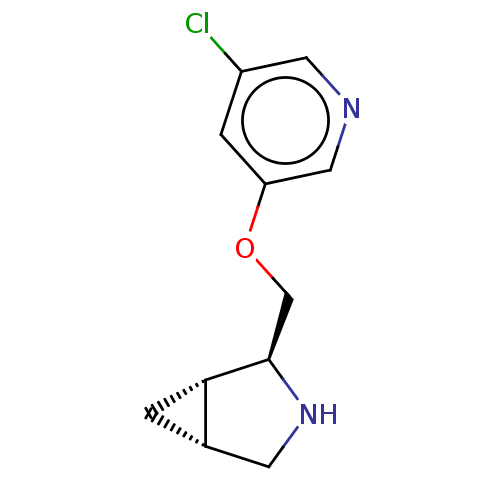
(CHEMBL4647198)Show SMILES Cl.[H][C@@]12C[C@]1([H])[C@@H](COc1cncc(Cl)c1)NC2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-8-2-9(5-13-4-8)15-6-11-10-1-7(10)3-14-11;/h2,4-5,7,10-11,14H,1,3,6H2;1H/t7-,10-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538047
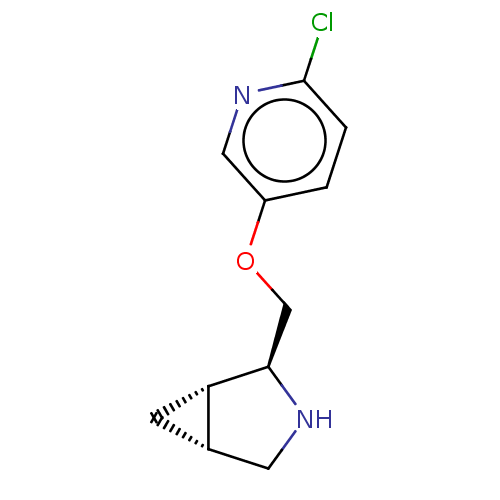
(CHEMBL4647019)Show SMILES [H][C@@]12C[C@]1([H])[C@@H](COc1ccc(Cl)nc1)NC2 |r| Show InChI InChI=1S/C11H13ClN2O/c12-11-2-1-8(5-14-11)15-6-10-9-3-7(9)4-13-10/h1-2,5,7,9-10,13H,3-4,6H2/t7-,9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538027
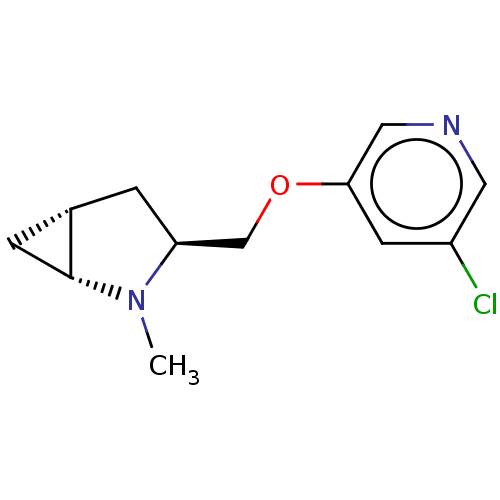
(CHEMBL4644169)Show SMILES [H][C@]12C[C@@]1([H])N(C)[C@H](COc1cncc(Cl)c1)C2 |r| Show InChI InChI=1S/C12H15ClN2O/c1-15-10(2-8-3-12(8)15)7-16-11-4-9(13)5-14-6-11/h4-6,8,10,12H,2-3,7H2,1H3/t8-,10-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538033
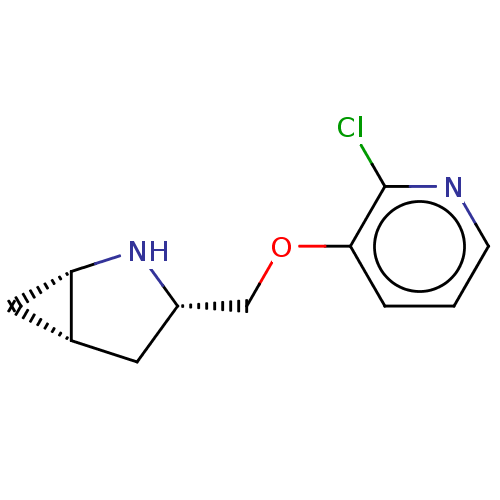
(CHEMBL4648557)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1cccnc1Cl)C2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-11-10(2-1-3-13-11)15-6-8-4-7-5-9(7)14-8;/h1-3,7-9,14H,4-6H2;1H/t7-,8+,9+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538030
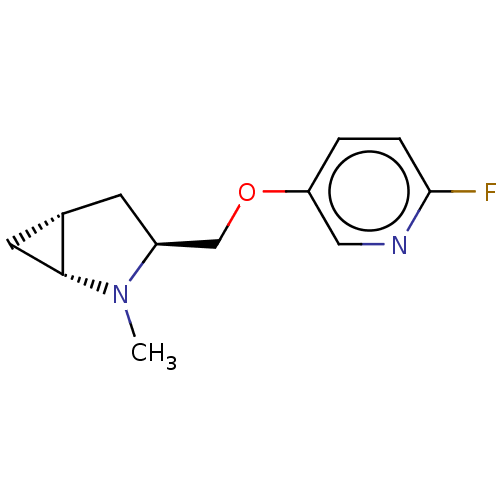
(CHEMBL4638919)Show SMILES Cl.[H][C@]12C[C@@]1([H])N(C)[C@H](COc1ccc(F)nc1)C2 |r| Show InChI InChI=1S/C12H15FN2O.ClH/c1-15-9(4-8-5-11(8)15)7-16-10-2-3-12(13)14-6-10;/h2-3,6,8-9,11H,4-5,7H2,1H3;1H/t8-,9-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538038
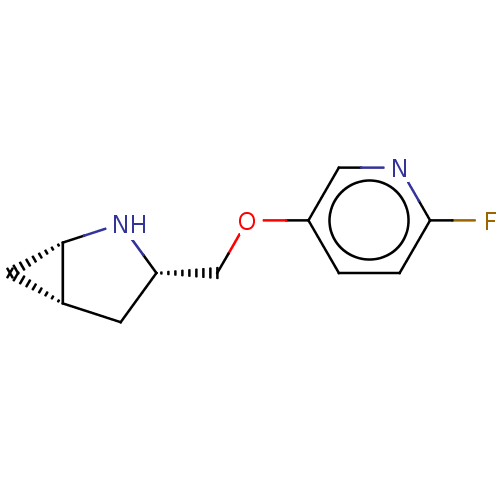
(CHEMBL4648098)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1ccc(F)nc1)C2 |r| Show InChI InChI=1S/C11H13FN2O.ClH/c12-11-2-1-9(5-13-11)15-6-8-3-7-4-10(7)14-8;/h1-2,5,7-8,10,14H,3-4,6H2;1H/t7-,8+,10+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538036
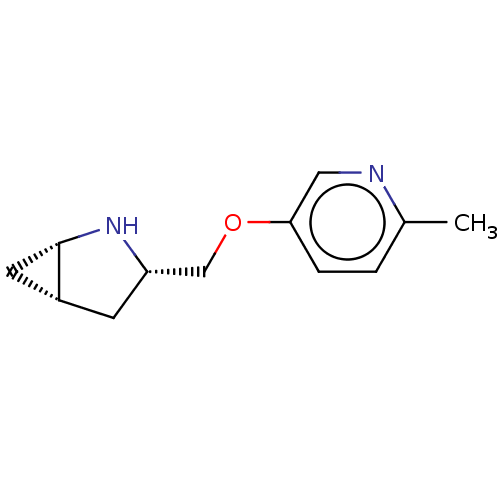
(CHEMBL4634253)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1ccc(C)nc1)C2 |r| Show InChI InChI=1S/C12H16N2O.ClH/c1-8-2-3-11(6-13-8)15-7-10-4-9-5-12(9)14-10;/h2-3,6,9-10,12,14H,4-5,7H2,1H3;1H/t9-,10+,12+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538063
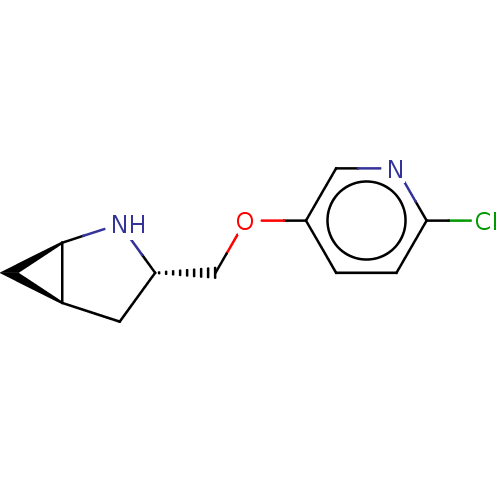
(CHEMBL4642118)Show SMILES Cl.[H][C@]12C[C@@]1([H])N[C@H](COc1ccc(Cl)nc1)C2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-11-2-1-9(5-13-11)15-6-8-3-7-4-10(7)14-8;/h1-2,5,7-8,10,14H,3-4,6H2;1H/t7-,8-,10+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518688

(US11116764, Compound 1 | US11253514, Compound 1 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested at MDS pharma services and Novascreen according to the following procedures.Materials and Methods:Receptor source: Human recombin... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q50BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518688

(US11116764, Compound 1 | US11253514, Compound 1 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Example 2: Determination of Ki Value at 5-HT6 ReceptorCompound was tested at MDS pharma services and Novascreen according to the following procedures... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W66PZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518688

(US11116764, Compound 1 | US11253514, Compound 1 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: Incubation conditions: Reactions were carried out in 50 mM Tris-HCl (pH 7.4) containing 10 mM MgCl2, 0.5 mM EDTA for 60 minutes at 37° C. Th... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B639J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345760

(1-(Benzenesulfonyl)-5-(piperazin-1-yl-methyl)-1H-i...)Show InChI InChI=1S/C19H21N3O2S/c23-25(24,18-4-2-1-3-5-18)22-11-8-17-14-16(6-7-19(17)22)15-21-12-9-20-10-13-21/h1-8,11,14,20H,9-10,12-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345777

(1-(2-Bromo benzenesulfonyl)-5-(4-ethylpiperazin-1-...)Show SMILES CCN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C21H24BrN3O2S/c1-2-23-11-13-24(14-12-23)16-17-7-8-20-18(15-17)9-10-25(20)28(26,27)21-6-4-3-5-19(21)22/h3-10,15H,2,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345758

(3-chloro-1-(4-fluoro benzenesulfonyl)-5-(piperazin...)Show SMILES Fc1ccc(cc1)S(=O)(=O)n1cc(Cl)c2cc(CN3CCNCC3)ccc12 Show InChI InChI=1S/C19H19ClFN3O2S/c20-18-13-24(27(25,26)16-4-2-15(21)3-5-16)19-6-1-14(11-17(18)19)12-23-9-7-22-8-10-23/h1-6,11,13,22H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538014
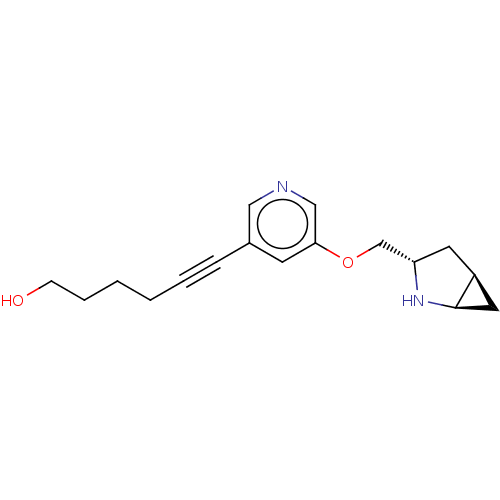
(CHEMBL4637076)Show SMILES OC(=O)C(O)=O.[H][C@]12C[C@@]1([H])N[C@H](COc1cncc(c1)C#CCCCCO)C2 |r| Show InChI InChI=1S/C17H22N2O2.C2H2O4/c20-6-4-2-1-3-5-13-7-16(11-18-10-13)21-12-15-8-14-9-17(14)19-15;3-1(4)2(5)6/h7,10-11,14-15,17,19-20H,1-2,4,6,8-9,12H2;(H,3,4)(H,5,6)/t14-,15-,17+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538035
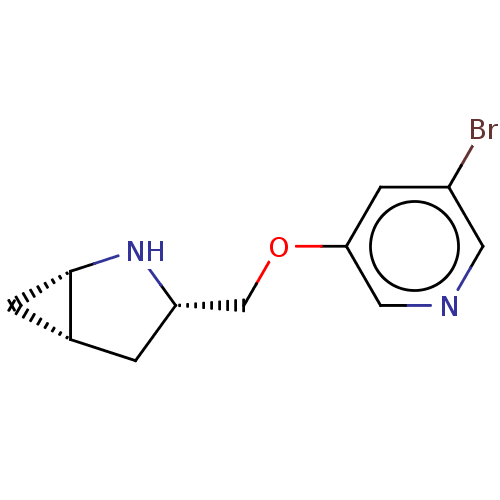
(CHEMBL4634110)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1cncc(Br)c1)C2 |r| Show InChI InChI=1S/C11H13BrN2O.ClH/c12-8-3-10(5-13-4-8)15-6-9-1-7-2-11(7)14-9;/h3-5,7,9,11,14H,1-2,6H2;1H/t7-,9+,11+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345754
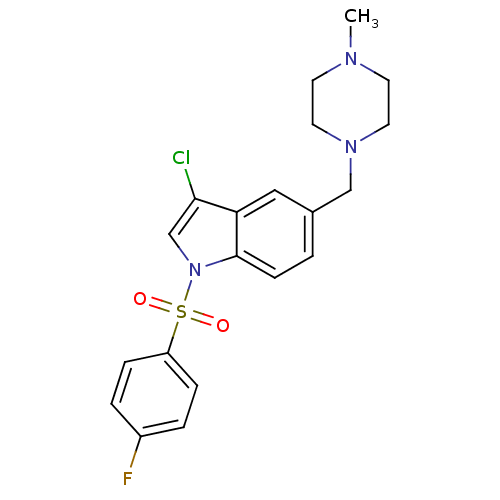
(3-chloro-1-(4-fluoro benzenesulfonyl)-5-(4-methylp...)Show SMILES CN1CCN(Cc2ccc3n(cc(Cl)c3c2)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C20H21ClFN3O2S/c1-23-8-10-24(11-9-23)13-15-2-7-20-18(12-15)19(21)14-25(20)28(26,27)17-5-3-16(22)4-6-17/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345763
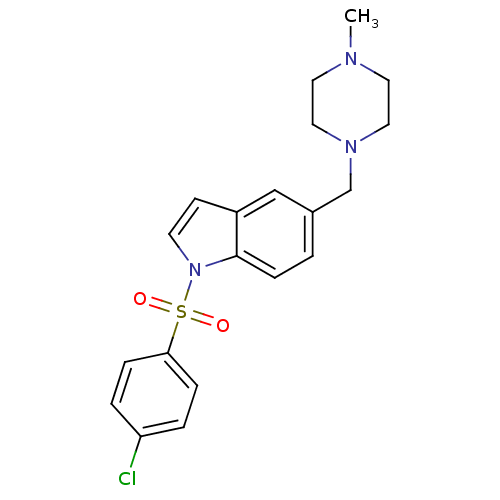
(1-(4-Chloro benzenesulfonyl)-5-(4-methylpiperazin-...)Show SMILES CN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C20H22ClN3O2S/c1-22-10-12-23(13-11-22)15-16-2-7-20-17(14-16)8-9-24(20)27(25,26)19-5-3-18(21)4-6-19/h2-9,14H,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345762

(1-(3-trifluoromethyl benzenesulfonyl)-5-(4-methylp...)Show SMILES CN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C21H22F3N3O2S/c1-25-9-11-26(12-10-25)15-16-5-6-20-17(13-16)7-8-27(20)30(28,29)19-4-2-3-18(14-19)21(22,23)24/h2-8,13-14H,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345772

(1-(4-Fluoro benzenesulfonyl)-5-(4-ethylpiperazin-1...)Show SMILES CCN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C21H24FN3O2S/c1-2-23-11-13-24(14-12-23)16-17-3-8-21-18(15-17)9-10-25(21)28(26,27)20-6-4-19(22)5-7-20/h3-10,15H,2,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345753

(3-Chloro-1-(2,4-difluorobenzenesulfonyl)-5-(4-meth...)Show SMILES CN1CCN(Cc2ccc3n(cc(Cl)c3c2)S(=O)(=O)c2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H20ClF2N3O2S/c1-24-6-8-25(9-7-24)12-14-2-4-19-16(10-14)17(21)13-26(19)29(27,28)20-5-3-15(22)11-18(20)23/h2-5,10-11,13H,6-9,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538029

(CHEMBL4648968)Show SMILES Cl.[H][C@]12C[C@@]1([H])N(C)[C@H](COc1ccc(Cl)nc1)C2 |r| Show InChI InChI=1S/C12H15ClN2O.ClH/c1-15-9(4-8-5-11(8)15)7-16-10-2-3-12(13)14-6-10;/h2-3,6,8-9,11H,4-5,7H2,1H3;1H/t8-,9-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334248
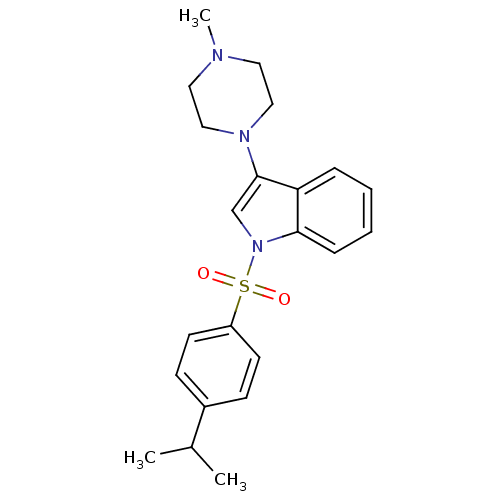
(1-(4-Isopropylbenzenesulfonyl)-3-(4-methylpiperazi...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)n1cc(N2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C22H27N3O2S/c1-17(2)18-8-10-19(11-9-18)28(26,27)25-16-22(20-6-4-5-7-21(20)25)24-14-12-23(3)13-15-24/h4-11,16-17H,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells by glass fiber filtration assay |
Bioorg Med Chem Lett 21: 346-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.001
BindingDB Entry DOI: 10.7270/Q2P84C5W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538041

(CHEMBL4642769)Show InChI InChI=1S/C12H16N2O.ClH/c1-12-6-9(12)5-10(14-12)8-15-11-3-2-4-13-7-11;/h2-4,7,9-10,14H,5-6,8H2,1H3;1H/t9-,10?,12+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518702
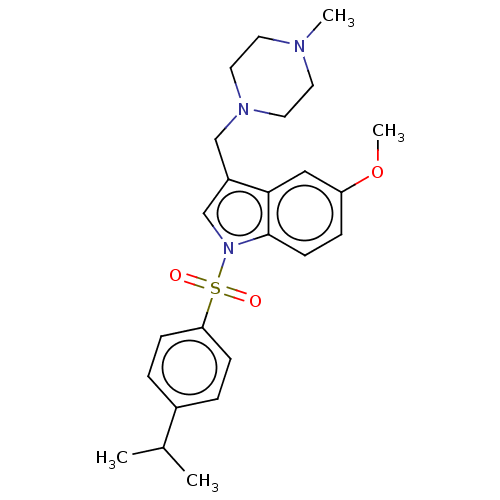
(US11116764, Compound 3 | US11253514, Compound 3 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested at MDS pharma services and Novascreen according to the following procedures.Materials and Methods:Receptor source: Human recombin... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q50BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518702

(US11116764, Compound 3 | US11253514, Compound 3 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: Incubation conditions: Reactions were carried out in 50 mM Tris-HCl (pH 7.4) containing 10 mM MgCl2, 0.5 mM EDTA for 60 minutes at 37° C. Th... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B639J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518702

(US11116764, Compound 3 | US11253514, Compound 3 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Example 2: Determination of Ki Value at 5-HT6 ReceptorCompound was tested at MDS pharma services and Novascreen according to the following procedures... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W66PZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345769

(1-Benzenesulfonyl-5-(4-methylpiperazin-1-yl-methyl...)Show SMILES CN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2ccccc2)CC1 Show InChI InChI=1S/C20H23N3O2S/c1-21-11-13-22(14-12-21)16-17-7-8-20-18(15-17)9-10-23(20)26(24,25)19-5-3-2-4-6-19/h2-10,15H,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538064

(CHEMBL4642865)Show SMILES Cl.[H][C@]12C[C@@]1([H])N[C@H](COc1ccc(F)nc1)C2 |r| Show InChI InChI=1S/C11H13FN2O.ClH/c12-11-2-1-9(5-13-11)15-6-8-3-7-4-10(7)14-8;/h1-2,5,7-8,10,14H,3-4,6H2;1H/t7-,8-,10+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345756
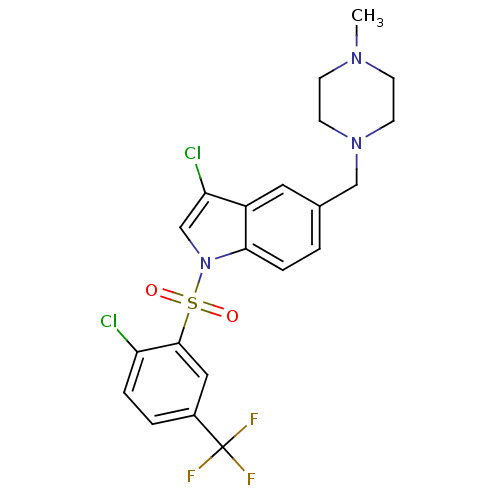
(1-(2-Chloro-5-trifluoromethyl benzenesulfonyl)-3-c...)Show SMILES CN1CCN(Cc2ccc3n(cc(Cl)c3c2)S(=O)(=O)c2cc(ccc2Cl)C(F)(F)F)CC1 Show InChI InChI=1S/C21H20Cl2F3N3O2S/c1-27-6-8-28(9-7-27)12-14-2-5-19-16(10-14)18(23)13-29(19)32(30,31)20-11-15(21(24,25)26)3-4-17(20)22/h2-5,10-11,13H,6-9,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538028
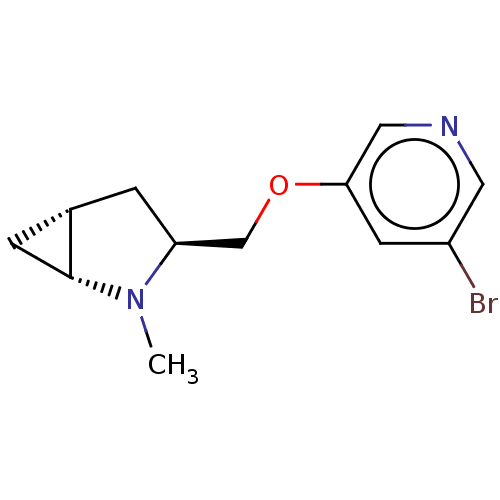
(CHEMBL4634727)Show SMILES Cl.[H][C@]12C[C@@]1([H])N(C)[C@H](COc1cncc(Br)c1)C2 |r| Show InChI InChI=1S/C12H15BrN2O.ClH/c1-15-10(2-8-3-12(8)15)7-16-11-4-9(13)5-14-6-11;/h4-6,8,10,12H,2-3,7H2,1H3;1H/t8-,10-,12+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538039

(CHEMBL4637371)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1ccc(OC)nc1)C2 |r| Show InChI InChI=1S/C12H16N2O2.ClH/c1-15-12-3-2-10(6-13-12)16-7-9-4-8-5-11(8)14-9;/h2-3,6,8-9,11,14H,4-5,7H2,1H3;1H/t8-,9+,11+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518701

(US11116764, Compound 2 | US11253514, Compound 2 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested at MDS pharma services and Novascreen according to the following procedures.Materials and Methods:Receptor source: Human recombin... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q50BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518701

(US11116764, Compound 2 | US11253514, Compound 2 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Example 2: Determination of Ki Value at 5-HT6 ReceptorCompound was tested at MDS pharma services and Novascreen according to the following procedures... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W66PZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM518701

(US11116764, Compound 2 | US11253514, Compound 2 | ...)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: Incubation conditions: Reactions were carried out in 50 mM Tris-HCl (pH 7.4) containing 10 mM MgCl2, 0.5 mM EDTA for 60 minutes at 37° C. Th... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B639J |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538022

(CHEMBL4649539)Show SMILES [H][C@]12C[C@@]1([H])N(C)[C@H](COc1cccnc1)C2 |r| Show InChI InChI=1S/C12H16N2O/c1-14-10(5-9-6-12(9)14)8-15-11-3-2-4-13-7-11/h2-4,7,9-10,12H,5-6,8H2,1H3/t9-,10-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345776
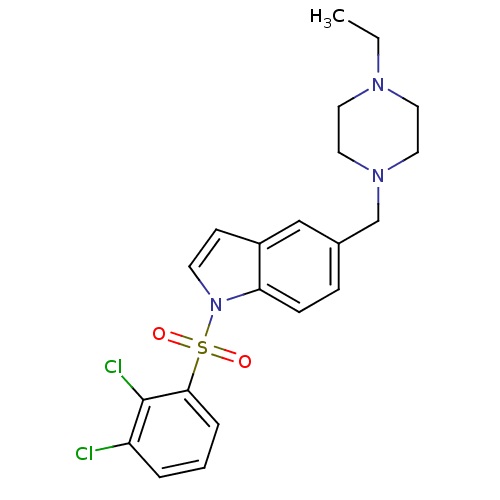
(1-(2,3-Dichlorobenzenesulfonyl)-5-(4-ethylpiperazi...)Show SMILES CCN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2cccc(Cl)c2Cl)CC1 Show InChI InChI=1S/C21H23Cl2N3O2S/c1-2-24-10-12-25(13-11-24)15-16-6-7-19-17(14-16)8-9-26(19)29(27,28)20-5-3-4-18(22)21(20)23/h3-9,14H,2,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345757

(3-Chloro-1-(2,4-difluoro benzenesulfonyl)-5-(piper...)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)n1cc(Cl)c2cc(CN3CCNCC3)ccc12 Show InChI InChI=1S/C19H18ClF2N3O2S/c20-16-12-25(28(26,27)19-4-2-14(21)10-17(19)22)18-3-1-13(9-15(16)18)11-24-7-5-23-6-8-24/h1-4,9-10,12,23H,5-8,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345775
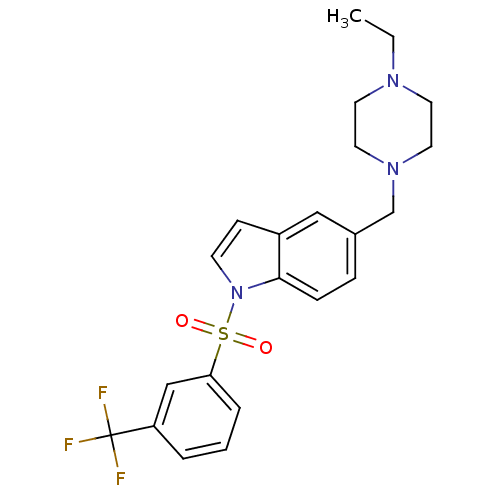
(1-(3-trifluoromethyl benzenesulfonyl)-5-(4-ethylpi...)Show SMILES CCN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C22H24F3N3O2S/c1-2-26-10-12-27(13-11-26)16-17-6-7-21-18(14-17)8-9-28(21)31(29,30)20-5-3-4-19(15-20)22(23,24)25/h3-9,14-15H,2,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345774

(1-(4-Isopropyl benzenesulfonyl)-5-(4-ethylpiperazi...)Show SMILES CCN1CCN(Cc2ccc3n(ccc3c2)S(=O)(=O)c2ccc(cc2)C(C)C)CC1 Show InChI InChI=1S/C24H31N3O2S/c1-4-25-13-15-26(16-14-25)18-20-5-10-24-22(17-20)11-12-27(24)30(28,29)23-8-6-21(7-9-23)19(2)3/h5-12,17,19H,4,13-16,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538062
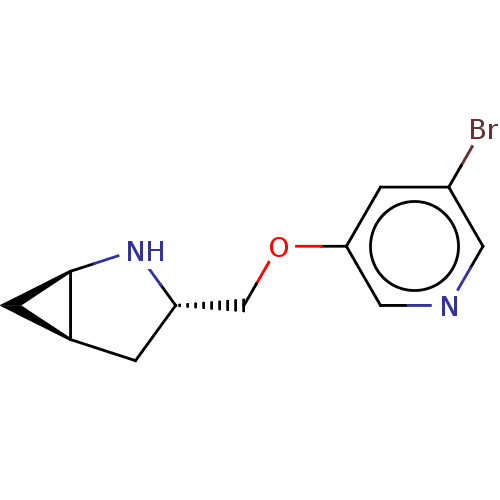
(CHEMBL4637698)Show SMILES OC(C(O)C(O)=O)C(O)=O.[H][C@]12C[C@@]1([H])N[C@H](COc1cncc(Br)c1)C2 |r| Show InChI InChI=1S/C11H13BrN2O.C4H6O6/c12-8-3-10(5-13-4-8)15-6-9-1-7-2-11(7)14-9;5-1(3(7)8)2(6)4(9)10/h3-5,7,9,11,14H,1-2,6H2;1-2,5-6H,(H,7,8)(H,9,10)/t7-,9-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538037

(CHEMBL4645367)Show SMILES Cl.[H][C@@]12C[C@]1([H])N[C@H](COc1ccc(Cl)nc1)C2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-11-2-1-9(5-13-11)15-6-8-3-7-4-10(7)14-8;/h1-2,5,7-8,10,14H,3-4,6H2;1H/t7-,8+,10+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50345761
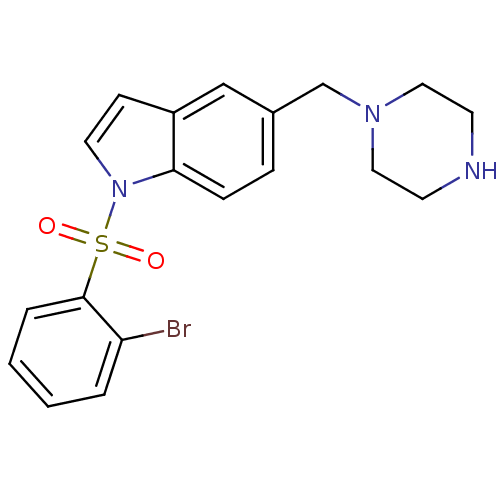
(1-(2-Bromo benzenesulfonyl)-5-(piperazin-1-yl-meth...)Show SMILES Brc1ccccc1S(=O)(=O)n1ccc2cc(CN3CCNCC3)ccc12 Show InChI InChI=1S/C19H20BrN3O2S/c20-17-3-1-2-4-19(17)26(24,25)23-10-7-16-13-15(5-6-18(16)23)14-22-11-8-21-9-12-22/h1-7,10,13,21H,8-9,11-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells |
ACS Med Chem Lett 1: 340-344 (2010)
Article DOI: 10.1021/ml100101u
BindingDB Entry DOI: 10.7270/Q2CR5TPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334254
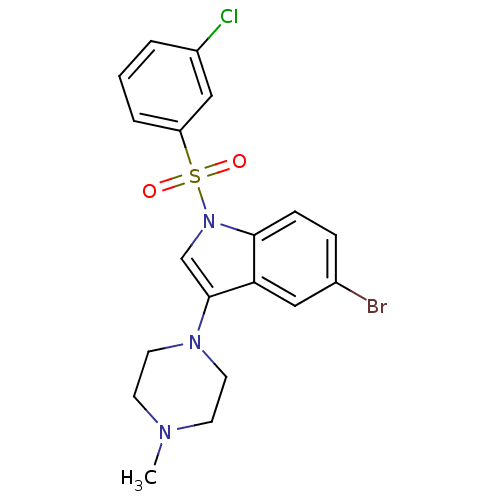
(5-Bromo-1-(3-chlorobenzenesulfonyl)-3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cn(c2ccc(Br)cc12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H19BrClN3O2S/c1-22-7-9-23(10-8-22)19-13-24(18-6-5-14(20)11-17(18)19)27(25,26)16-4-2-3-15(21)12-16/h2-6,11-13H,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells by glass fiber filtration assay |
Bioorg Med Chem Lett 21: 346-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.001
BindingDB Entry DOI: 10.7270/Q2P84C5W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538057
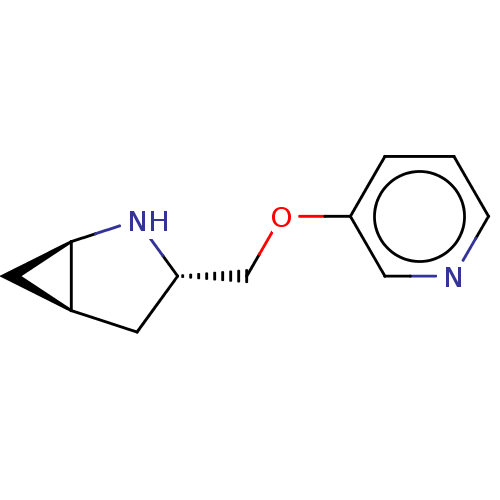
(CHEMBL4643942)Show SMILES Cl.[H][C@]12C[C@@]1([H])N[C@H](COc1cccnc1)C2 |r| Show InChI InChI=1S/C11H14N2O.ClH/c1-2-10(6-12-3-1)14-7-9-4-8-5-11(8)13-9;/h1-3,6,8-9,11,13H,4-5,7H2;1H/t8-,9-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50538046

(CHEMBL4647198)Show SMILES Cl.[H][C@@]12C[C@]1([H])[C@@H](COc1cncc(Cl)c1)NC2 |r| Show InChI InChI=1S/C11H13ClN2O.ClH/c12-8-2-9(5-13-4-8)15-6-11-10-1-7(10)3-14-11;/h2,4-5,7,10-11,14H,1,3,6H2;1H/t7-,10-,11+;/m0./s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha3beta4 nAChR expressed in IMR32 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data