| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Purine nucleoside phosphorylase |
|---|
| Ligand | BDBM50049958 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_162192 (CHEMBL873424) |
|---|
| Ki | 550±n/a nM |
|---|
| Citation |  Beauchamp, LM; Tuttle, JV; Rodriguez, ME; Sznaidman, ML Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J Med Chem39:949-56 (1996) [PubMed] Article Beauchamp, LM; Tuttle, JV; Rodriguez, ME; Sznaidman, ML Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J Med Chem39:949-56 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Purine nucleoside phosphorylase |
|---|
| Name: | Purine nucleoside phosphorylase |
|---|
| Synonyms: | Inosine phosphorylase | Inosine-guanosine phosphorylase | NP | PNP | PNPH_HUMAN | Purine nucleoside phosphorylase (PNPase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 32119.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 289 |
|---|
| Sequence: | MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRST
VPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGL
NPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQ
MGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSL
ITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS
|
|
|
|---|
| BDBM50049958 |
|---|
| n/a |
|---|
| Name | BDBM50049958 |
|---|
| Synonyms: | CHEMBL172306 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,3]triazolo[4,5-d]pyrimidin-2-ylmethoxy)-pentyl]-phosphonic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H17N6O5P |
|---|
| Mol. Mass. | 332.2529 |
|---|
| SMILES | Nc1nc2nn(COCCCCCP(O)(O)=O)nc2c(=O)[nH]1 |
|---|
| Structure | 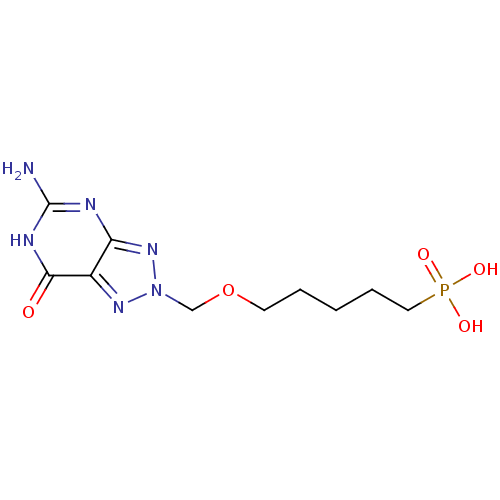
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Beauchamp, LM; Tuttle, JV; Rodriguez, ME; Sznaidman, ML Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J Med Chem39:949-56 (1996) [PubMed] Article
Beauchamp, LM; Tuttle, JV; Rodriguez, ME; Sznaidman, ML Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J Med Chem39:949-56 (1996) [PubMed] Article