 Found 1856 hits with Last Name = 'rodriguez' and Initial = 'me'
Found 1856 hits with Last Name = 'rodriguez' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50033666

(2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...)Show SMILES Nc1nc2n(COCCOP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H13N5O9P2/c9-8-11-6-5(7(14)12-8)10-3-13(6)4-20-1-2-21-24(18,19)22-23(15,16)17/h3H,1-2,4H2,(H,18,19)(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049967

(CHEMBL174603 | [4-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C9H15N6O5P/c10-9-11-7-6(8(16)12-9)13-14-15(7)5-20-3-1-2-4-21(17,18)19/h1-5H2,(H2,17,18,19)(H3,10,11,12,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049963
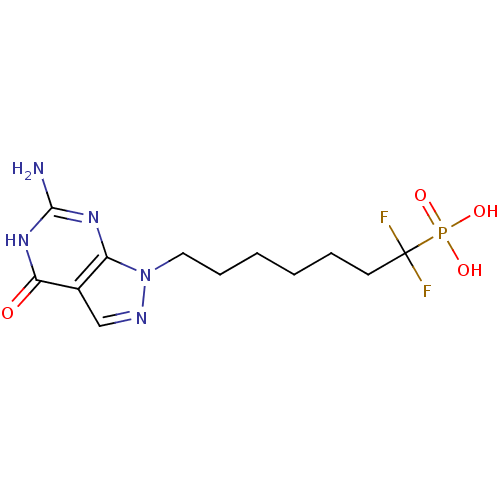
(CHEMBL368924 | [7-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show SMILES Nc1nc2n(CCCCCCC(F)(F)P(O)(O)=O)ncc2c(=O)[nH]1 Show InChI InChI=1S/C12H18F2N5O4P/c13-12(14,24(21,22)23)5-3-1-2-4-6-19-9-8(7-16-19)10(20)18-11(15)17-9/h7H,1-6H2,(H2,21,22,23)(H3,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049969
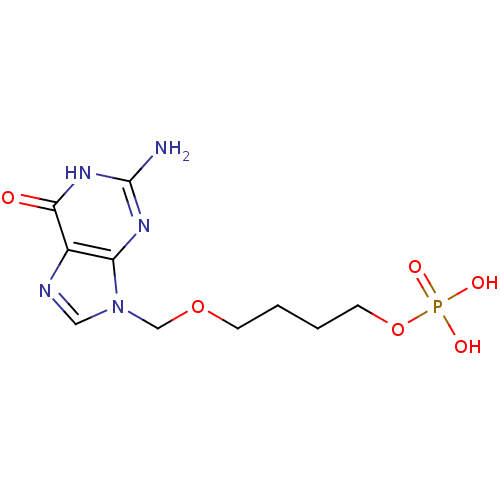
(CHEMBL172316 | Phosphoric acid mono-[4-(2-amino-6-...)Show InChI InChI=1S/C10H16N5O6P/c11-10-13-8-7(9(16)14-10)12-5-15(8)6-20-3-1-2-4-21-22(17,18)19/h5H,1-4,6H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049966
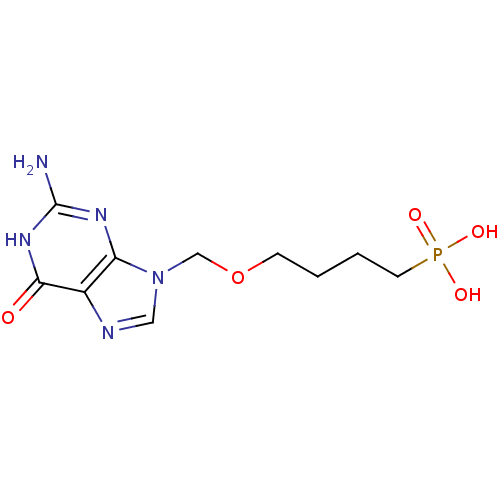
(CHEMBL177948 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C10H16N5O5P/c11-10-13-8-7(9(16)14-10)12-5-15(8)6-20-3-1-2-4-21(17,18)19/h5H,1-4,6H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049971

(CHEMBL177190 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...)Show SMILES Nc1nc2n(CCCCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18F2N5O4P/c13-12(14,24(21,22)23)5-3-1-2-4-6-19-7-16-8-9(19)17-11(15)18-10(8)20/h7H,1-6H2,(H2,21,22,23)(H3,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049954

(CHEMBL369052 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C9H15N6O4P/c10-9-11-7-6(8(16)12-9)13-14-15(7)4-2-1-3-5-20(17,18)19/h1-5H2,(H2,17,18,19)(H3,10,11,12,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049959
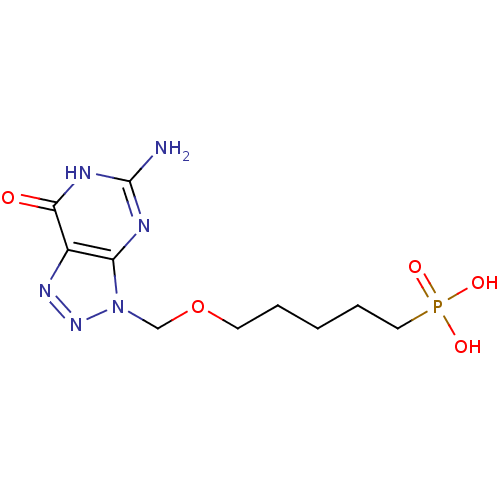
(CHEMBL173142 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O5P/c11-10-12-8-7(9(17)13-10)14-15-16(8)6-21-4-2-1-3-5-22(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049972

(CHEMBL176448 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C11H18N5O5P/c12-11-14-9-8(10(17)15-11)13-6-16(9)7-21-4-2-1-3-5-22(18,19)20/h6H,1-5,7H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049957
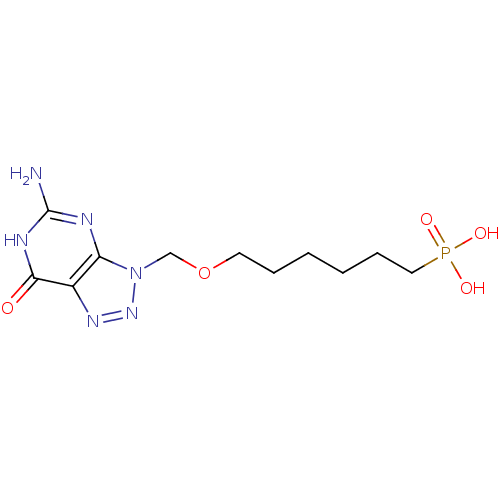
(CHEMBL366963 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C11H19N6O5P/c12-11-13-9-8(10(18)14-11)15-16-17(9)7-22-5-3-1-2-4-6-23(19,20)21/h1-7H2,(H2,19,20,21)(H3,12,13,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049968

(CHEMBL177945 | [6-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C12H20N5O5P/c13-12-15-10-9(11(18)16-12)14-7-17(10)8-22-5-3-1-2-4-6-23(19,20)21/h7H,1-6,8H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049955
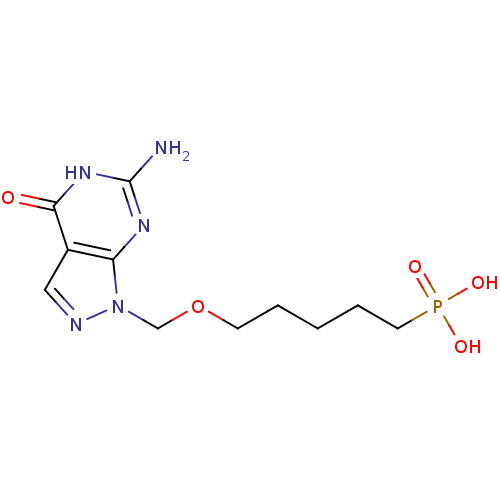
(CHEMBL175362 | [5-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C11H18N5O5P/c12-11-14-9-8(10(17)15-11)6-13-16(9)7-21-4-2-1-3-5-22(18,19)20/h6H,1-5,7H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049960
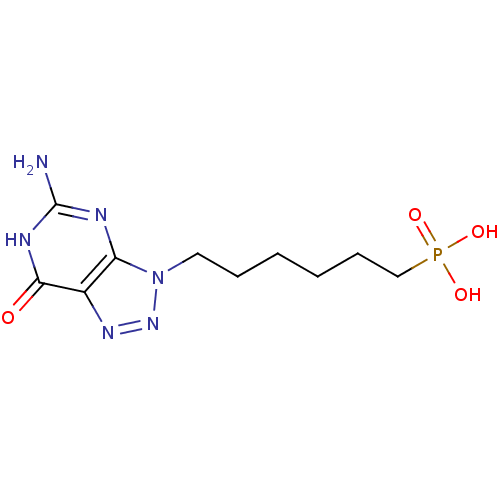
(CHEMBL368064 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O4P/c11-10-12-8-7(9(17)13-10)14-15-16(8)5-3-1-2-4-6-21(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049961
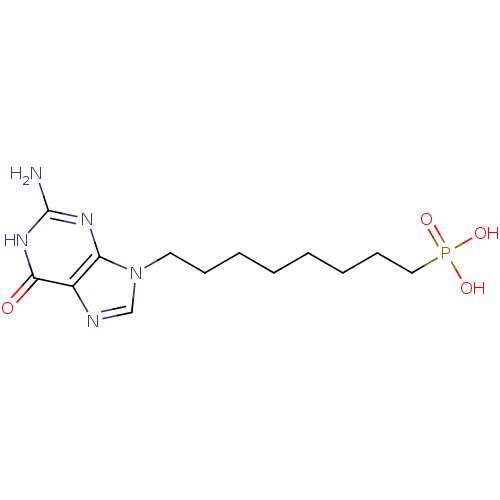
(CHEMBL354409 | [8-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C13H22N5O4P/c14-13-16-11-10(12(19)17-13)15-9-18(11)7-5-3-1-2-4-6-8-23(20,21)22/h9H,1-8H2,(H2,20,21,22)(H3,14,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049962
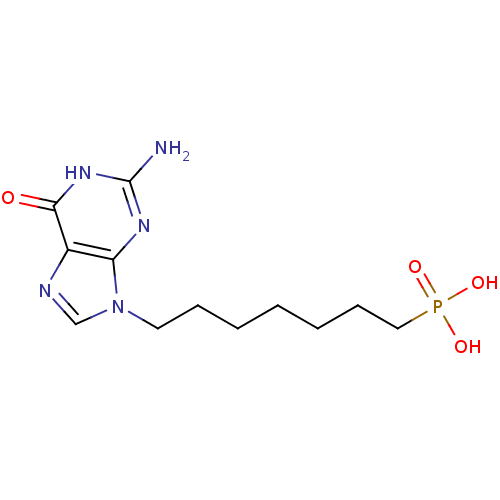
(CHEMBL172844 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C12H20N5O4P/c13-12-15-10-9(11(18)16-12)14-8-17(10)6-4-2-1-3-5-7-22(19,20)21/h8H,1-7H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049958
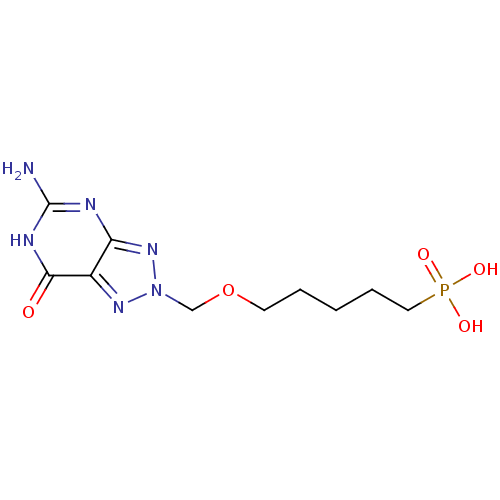
(CHEMBL172306 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O5P/c11-10-12-8-7(9(17)13-10)14-16(15-8)6-21-4-2-1-3-5-22(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049970
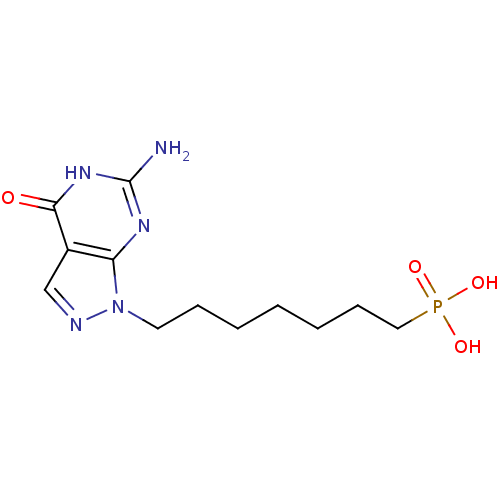
(CHEMBL176480 | TCMDC-137339 | [7-(6-Amino-4-oxo-4,...)Show InChI InChI=1S/C12H20N5O4P/c13-12-15-10-9(11(18)16-12)8-14-17(10)6-4-2-1-3-5-7-22(19,20)21/h8H,1-7H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049964
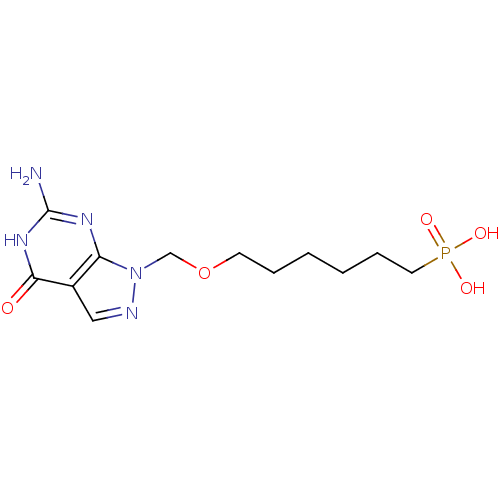
(CHEMBL173083 | [6-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C12H20N5O5P/c13-12-15-10-9(11(18)16-12)7-14-17(10)8-22-5-3-1-2-4-6-23(19,20)21/h7H,1-6,8H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049956
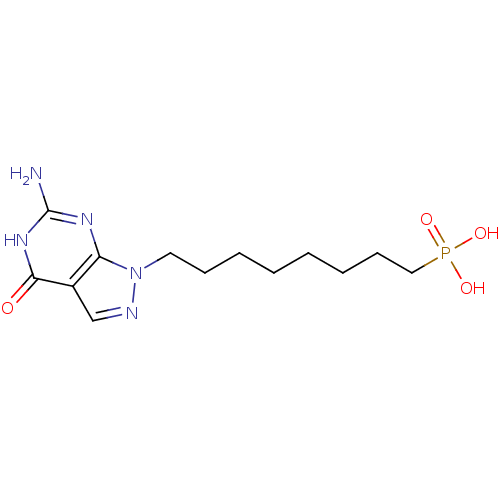
(CHEMBL176649 | [8-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C13H22N5O4P/c14-13-16-11-10(12(19)17-13)9-15-18(11)7-5-3-1-2-4-6-8-23(20,21)22/h9H,1-8H2,(H2,20,21,22)(H3,14,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049965
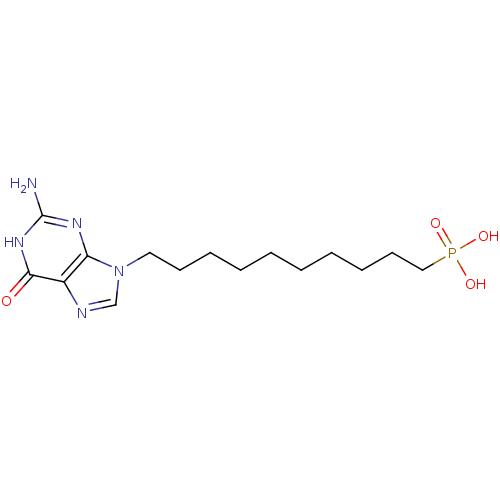
(CHEMBL176447 | [10-(2-Amino-6-oxo-1,6-dihydro-puri...)Show InChI InChI=1S/C15H26N5O4P/c16-15-18-13-12(14(21)19-15)17-11-20(13)9-7-5-3-1-2-4-6-8-10-25(22,23)24/h11H,1-10H2,(H2,22,23,24)(H3,16,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763
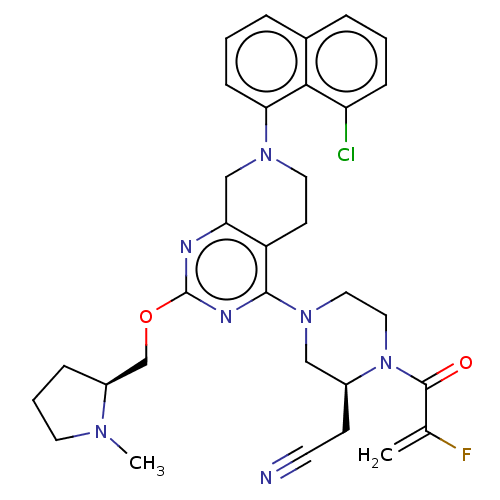
(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant KRAS G12C mutant (unknown origin) assessed as rate of inactivation by LC-MS analysis |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049973
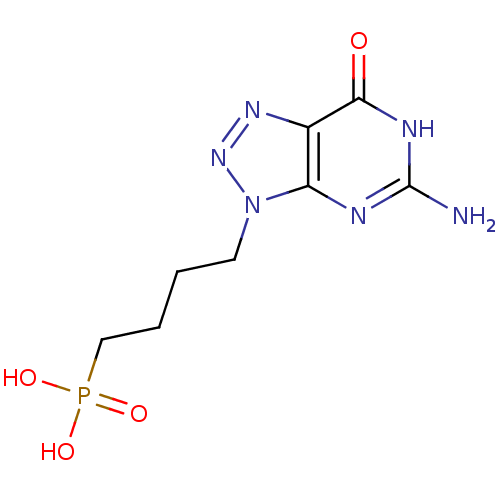
(CHEMBL175305 | [4-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C8H13N6O4P/c9-8-10-6-5(7(15)11-8)12-13-14(6)3-1-2-4-19(16,17)18/h1-4H2,(H2,16,17,18)(H3,9,10,11,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617349

(US20230279025, Example 465)Show SMILES CCc1cccc2cccc(-c3ncc4c(nc(OC[C@@]56CCCN5C[C@H](F)C6)nc4c3F)N3CC4CCC(C3)N4)c12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617357

(US20230279025, Example 469)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@]45C[C@@H](F)CN4CCCC5)nc3c2F)N2CC3CCC(C2)N3)c2c(C#C)c(F)ccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617363

(US20230279025, Example 482)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@]45CCCN4C[C@@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(C#C)c(F)ccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617323
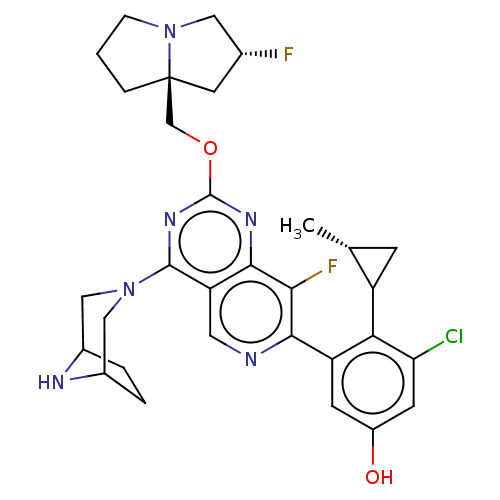
(US20230279025, Example 452 | US20230279025, Exampl...)Show SMILES C[C@@H]1CC1c1c(Cl)cc(O)cc1-c1ncc2c(nc(OC[C@@]34CCCN3C[C@H](F)C4)nc2c1F)N1CC2CCC(C1)N2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617355
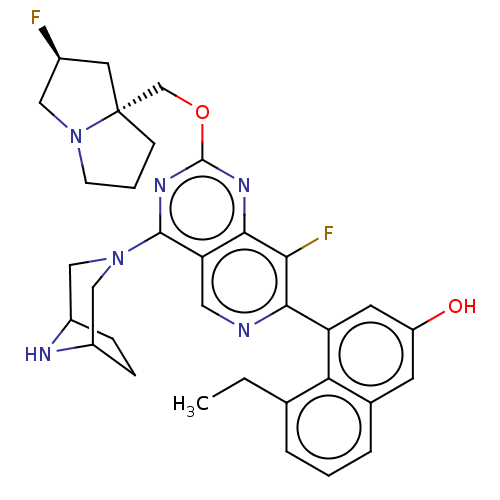
(US20230279025, Example 468)Show SMILES CCc1cccc2cc(O)cc(-c3ncc4c(nc(OC[C@]56CCCN5C[C@@H](F)C6)nc4c3F)N3CC4CCC(C3)N4)c12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573400

(US11453683, Example 259 | US20230279025, Example 4...)Show SMILES CCc1cccc2cc(O)cc(-c3ncc4c(nc(OC[C@@]56CCCN5C[C@H](F)C6)nc4c3F)N3CC4CCC(C3)N4)c12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579601
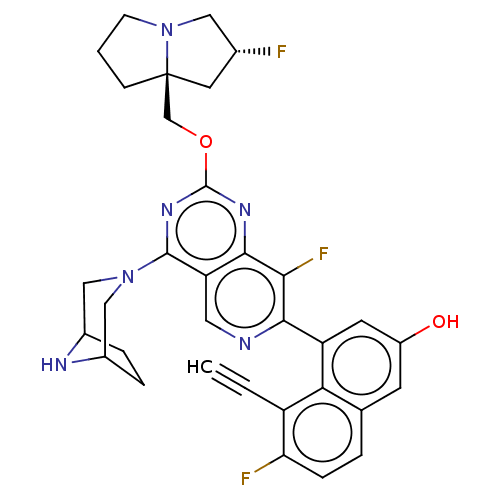
(CHEMBL4858364 | US11453683, Example 252 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(C#C)c(F)ccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617351
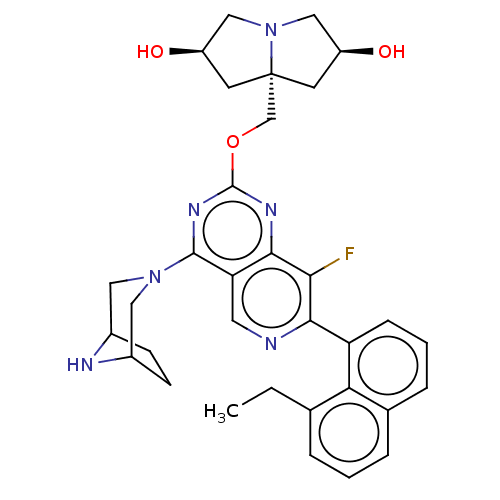
(US20230279025, Example 466)Show SMILES CCc1cccc2cccc(-c3ncc4c(nc(OC[C@]56C[C@@H](O)CN5C[C@@H](O)C6)nc4c3F)N3CC4CCC(C3)N4)c12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617347
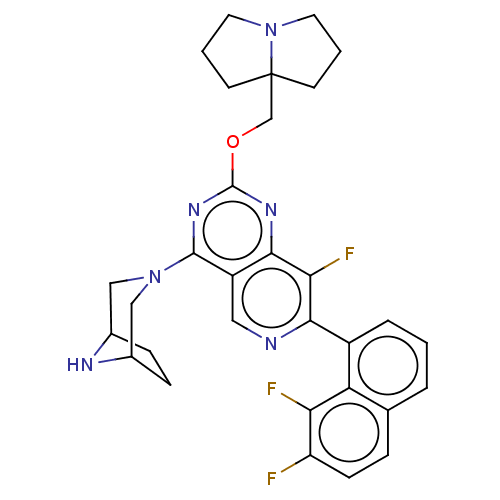
(US20230279025, Example 464)Show SMILES Fc1ccc2cccc(-c3ncc4c(nc(OCC56CCCN5CCC6)nc4c3F)N3CC4CCC(C3)N4)c2c1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617345
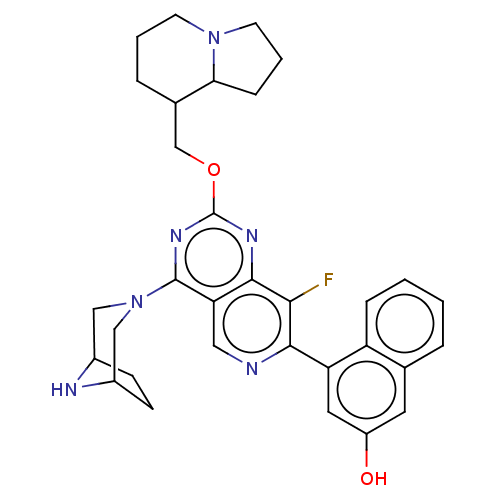
(US20230279025, Example 463)Show SMILES Oc1cc(-c2ncc3c(nc(OCC4CCCN5CCCC45)nc3c2F)N2CC3CCC(C2)N3)c2ccccc2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM617339

(US20230279025, Example 460)Show SMILES Fc1ccc2cccc(-c3ncc4c(nc(OCC56CCCN5CCC6)nc4c3F)N3CC4CCC(C3)N4)c2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM616938
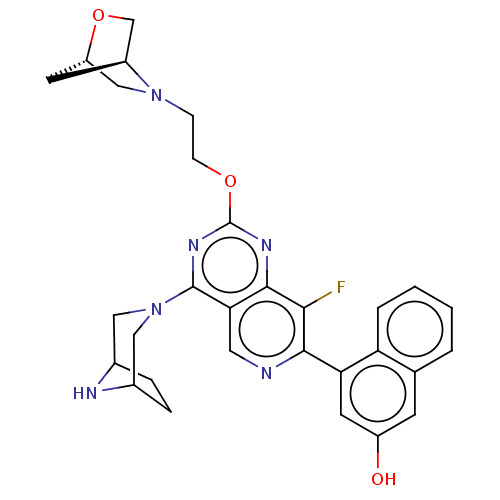
(US20230279025, Example 26 | US20230279025, Example...)Show SMILES Oc1cc(-c2ncc3c(nc(OCCN4C[C@@H]5C[C@H]4CO5)nc3c2F)N2CC3CCC(C2)N3)c2ccccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573426

(US11453683, Example 284 | US20230279025, Example 2...)Show SMILES Oc1cc(Cl)c(c(c1)-c1ncc2c(nc(OC[C@@]34CCCN3C[C@H](F)C4)nc2c1F)N1CC2CCC(C1)N2)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573426

(US11453683, Example 284 | US20230279025, Example 2...)Show SMILES Oc1cc(Cl)c(c(c1)-c1ncc2c(nc(OC[C@@]34CCCN3C[C@H](F)C4)nc2c1F)N1CC2CCC(C1)N2)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579600
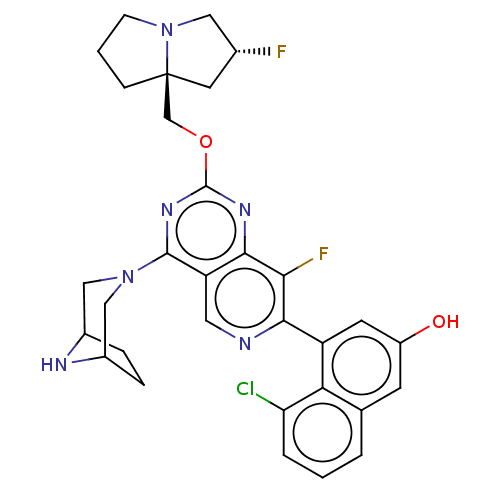
(CHEMBL4857438 | US11453683, Example 251 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(Cl)cccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579600

(CHEMBL4857438 | US11453683, Example 251 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(Cl)cccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579595

(CHEMBL4863339 | US11453683, Example 185 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OCC45CCCN4CCC5)nc3c2F)N2CC3CCC(C2)N3)c2c(cccc2c1)C#C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579595

(CHEMBL4863339 | US11453683, Example 185 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OCC45CCCN4CCC5)nc3c2F)N2CC3CCC(C2)N3)c2c(cccc2c1)C#C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579601

(CHEMBL4858364 | US11453683, Example 252 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(C#C)c(F)ccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579594

(CHEMBL4859236 | US11453683, Example 36 | US2023027...)Show SMILES Oc1cc(-c2ncc3c(nc(OCC45CCCN4CCC5)nc3c2F)N2CC3CCC(C2)N3)c2c(Cl)cccc2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573484

(US11453683, Example 340 | US20230279025, Example 3...)Show SMILES Oc1ccc(OC(F)(F)F)c(c1)-c1ncc2c(nc(OCC34CCCN3C[C@H](F)C4)nc2c1F)N1CC2CCC(C1)N2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573433
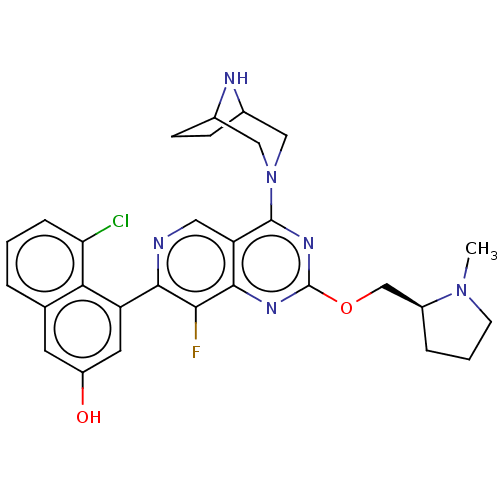
(US11453683, Example 63 | US20230279025, Example 63)Show SMILES CN1CCC[C@H]1COc1nc(N2CC3CCC(C2)N3)c2cnc(c(F)c2n1)-c1cc(O)cc2cccc(Cl)c12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579594

(CHEMBL4859236 | US11453683, Example 36 | US2023027...)Show SMILES Oc1cc(-c2ncc3c(nc(OCC45CCCN4CCC5)nc3c2F)N2CC3CCC(C2)N3)c2c(Cl)cccc2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579597

(CHEMBL4876040 | US11453683, Example 243 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(cccc2c1)C#C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM50579601

(CHEMBL4858364 | US11453683, Example 252 | US202302...)Show SMILES Oc1cc(-c2ncc3c(nc(OC[C@@]45CCCN4C[C@H](F)C5)nc3c2F)N2CC3CCC(C2)N3)c2c(C#C)c(F)ccc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573644

(US11453683, Example 439 | US20230279025, Example 4...)Show SMILES CN(C)C(=O)OC[C@H]1CC[C@@]2(COc3nc(N4CC5CCC(C4)N5)c4cnc(c(F)c4n3)-c3cccc4ccc(F)c(C#C)c34)CCCN12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573484

(US11453683, Example 340 | US20230279025, Example 3...)Show SMILES Oc1ccc(OC(F)(F)F)c(c1)-c1ncc2c(nc(OCC34CCCN3C[C@H](F)C4)nc2c1F)N1CC2CCC(C1)N2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QR5281 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12D]
(Homo sapiens (Human)) | BDBM573644

(US11453683, Example 439 | US20230279025, Example 4...)Show SMILES CN(C)C(=O)OC[C@H]1CC[C@@]2(COc3nc(N4CC5CCC(C4)N5)c4cnc(c(F)c4n3)-c3cccc4ccc(F)c(C#C)c34)CCCN12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PR807K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data