| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Retinoic acid receptor beta |
|---|
| Ligand | BDBM50082042 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_195482 (CHEMBL798904) |
|---|
| EC50 | 1004±n/a nM |
|---|
| Citation |  Zacheis, D; Dhar, A; Lu, S; Madler, MM; Klucik, J; Brown, CW; Liu, S; Clement, F; Subramanian, S; Weerasekare, GM; Berlin, KD; Gold, MA; Houck, JR; Fountain, KR; Benbrook, DM Heteroarotinoids inhibit head and neck cancer cell lines in vitro and in vivo through both RAR and RXR retinoic acid receptors. J Med Chem42:4434-45 (1999) [PubMed] Zacheis, D; Dhar, A; Lu, S; Madler, MM; Klucik, J; Brown, CW; Liu, S; Clement, F; Subramanian, S; Weerasekare, GM; Berlin, KD; Gold, MA; Houck, JR; Fountain, KR; Benbrook, DM Heteroarotinoids inhibit head and neck cancer cell lines in vitro and in vivo through both RAR and RXR retinoic acid receptors. J Med Chem42:4434-45 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Retinoic acid receptor beta |
|---|
| Name: | Retinoic acid receptor beta |
|---|
| Synonyms: | HAP | HBV-activated protein | NR1B2 | Nuclear receptor subfamily 1 group B member 2 | RAR-beta | RAR-epsilon | RARB | RARB_HUMAN | Retinoic acid receptor RXR-alpha/Retinoic acid receptor beta | Retinoic acid receptor beta | Retinoid receptor |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 50498.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1458015 |
|---|
| Residue: | 455 |
|---|
| Sequence: | MTTSGHACPVPAVNGHMTHYPATPYPLLFPPVIGGLSLPPLHGLHGHPPPSGCSTPSPAT
IETQSTSSEELVPSPPSPLPPPRVYKPCFVCQDKSSGYHYGVSACEGCKGFFRRSIQKNM
IYTCHRDKNCVINKVTRNRCQYCRLQKCFEVGMSKESVRNDRNKKKKETSKQECTESYEM
TAELDDLTEKIRKAHQETFPSLCQLGKYTTNSSADHRVRLDLGLWDKFSELATKCIIKIV
EFAKRLPGFTGLTIADQITLLKAACLDILILRICTRYTPEQDTMTFSDGLTLNRTQMHNA
GFGPLTDLVFTFANQLLPLEMDDTETGLLSAICLICGDRQDLEEPTKVDKLQEPLLEALK
IYIRKRRPSKPHMFPKILMKITDLRSISAKGAERVITLKMEIPGSMPPLIQEMLENSEGH
EPLTPSSSGNTAEHSPSISPSSVENSGVSQSPLVQ
|
|
|
|---|
| BDBM50082042 |
|---|
| n/a |
|---|
| Name | BDBM50082042 |
|---|
| Synonyms: | CHEMBL139536 | N-(2,2,4,4-Tetramethyl-thiochroman-6-yl)-terephthalamic acid methyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25NO3S |
|---|
| Mol. Mass. | 383.504 |
|---|
| SMILES | COC(=O)c1ccc(cc1)C(=O)Nc1ccc2SC(C)(C)CC(C)(C)c2c1 |
|---|
| Structure | 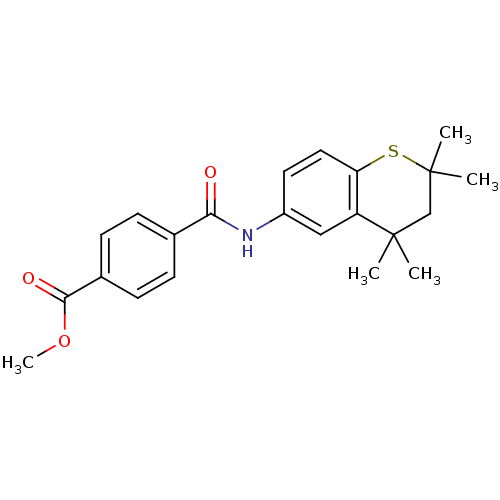
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zacheis, D; Dhar, A; Lu, S; Madler, MM; Klucik, J; Brown, CW; Liu, S; Clement, F; Subramanian, S; Weerasekare, GM; Berlin, KD; Gold, MA; Houck, JR; Fountain, KR; Benbrook, DM Heteroarotinoids inhibit head and neck cancer cell lines in vitro and in vivo through both RAR and RXR retinoic acid receptors. J Med Chem42:4434-45 (1999) [PubMed]
Zacheis, D; Dhar, A; Lu, S; Madler, MM; Klucik, J; Brown, CW; Liu, S; Clement, F; Subramanian, S; Weerasekare, GM; Berlin, KD; Gold, MA; Houck, JR; Fountain, KR; Benbrook, DM Heteroarotinoids inhibit head and neck cancer cell lines in vitro and in vivo through both RAR and RXR retinoic acid receptors. J Med Chem42:4434-45 (1999) [PubMed]