| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C8 |
|---|
| Ligand | BDBM50555364 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2051801 (CHEMBL4706802) |
|---|
| IC50 | >20000±n/a nM |
|---|
| Citation |  Kokkonda, S; Deng, X; White, KL; Coteron, JM; Marco, M; de Las Heras, L; White, J; El Mazouni, F; Tomchick, DR; Manjalanagara, K; Rudra, KR; Chen, G; Morizzi, J; Ryan, E; Kaminsky, W; Leroy, D; Martínez-Martínez, MS; Jimenez-Diaz, MB; Bazaga, SF; Angulo-Barturen, I; Waterson, D; Burrows, JN; Matthews, D; Charman, SA; Phillips, MA; Rathod, PK Tetrahydro-2-naphthyl and 2-Indanyl Triazolopyrimidines Targeting Plasmodium falciparum Dihydroorotate Dehydrogenase Display Potent and Selective Antimalarial Activity. J Med Chem59:5416-31 (2016) [PubMed] Article Kokkonda, S; Deng, X; White, KL; Coteron, JM; Marco, M; de Las Heras, L; White, J; El Mazouni, F; Tomchick, DR; Manjalanagara, K; Rudra, KR; Chen, G; Morizzi, J; Ryan, E; Kaminsky, W; Leroy, D; Martínez-Martínez, MS; Jimenez-Diaz, MB; Bazaga, SF; Angulo-Barturen, I; Waterson, D; Burrows, JN; Matthews, D; Charman, SA; Phillips, MA; Rathod, PK Tetrahydro-2-naphthyl and 2-Indanyl Triazolopyrimidines Targeting Plasmodium falciparum Dihydroorotate Dehydrogenase Display Potent and Selective Antimalarial Activity. J Med Chem59:5416-31 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C8 |
|---|
| Name: | Cytochrome P450 2C8 |
|---|
| Synonyms: | CP2C8_HUMAN | CYP2C8 | CYPIIC8 | Cytochrome P450 2C8 (CYP2C8) | P450 IIC2 | P450 MP-12/MP-20 | P450 form 1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55839.23 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10632 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MEPFVVLVLCLSFMLLFSLWRQSCRRRKLPPGPTPLPIIGNMLQIDVKDICKSFTNFSKV
YGPVFTVYFGMNPIVVFHGYEAVKEALIDNGEEFSGRGNSPISQRITKGLGIISSNGKRW
KEIRRFSLTTLRNFGMGKRSIEDRVQEEAHCLVEELRKTKASPCDPTFILGCAPCNVICS
VVFQKRFDYKDQNFLTLMKRFNENFRILNSPWIQVCNNFPLLIDCFPGTHNKVLKNVALT
RSYIREKVKEHQASLDVNNPRDFIDCFLIKMEQEKDNQKSEFNIENLVGTVADLFVAGTE
TTSTTLRYGLLLLLKHPEVTAKVQEEIDHVIGRHRSPCMQDRSHMPYTDAVVHEIQRYSD
LVPTGVPHAVTTDTKFRNYLIPKGTTIMALLTSVLHDDKEFPNPNIFDPGHFLDKNGNFK
KSDYFMPFSAGKRICAGEGLARMELFLFLTTILQNFNLKSVDDLKNLNTTAVTKGIVSLP
PSYQICFIPV
|
|
|
|---|
| BDBM50555364 |
|---|
| n/a |
|---|
| Name | BDBM50555364 |
|---|
| Synonyms: | CHEMBL4761666 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H18ClF2N5 |
|---|
| Mol. Mass. | 377.819 |
|---|
| SMILES | Cc1cc(NC2CCc3cc(Cl)ccc3C2)n2nc(nc2n1)C(C)(F)F |
|---|
| Structure | 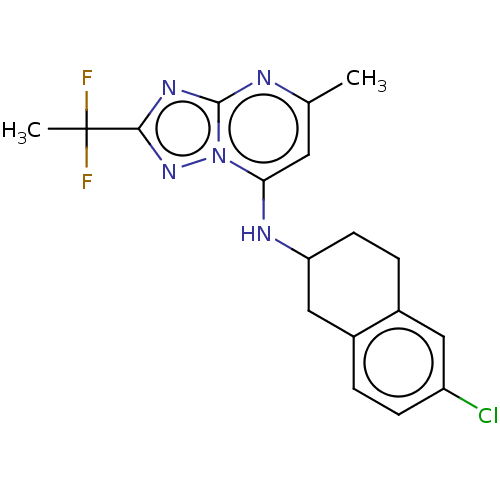
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kokkonda, S; Deng, X; White, KL; Coteron, JM; Marco, M; de Las Heras, L; White, J; El Mazouni, F; Tomchick, DR; Manjalanagara, K; Rudra, KR; Chen, G; Morizzi, J; Ryan, E; Kaminsky, W; Leroy, D; Martínez-Martínez, MS; Jimenez-Diaz, MB; Bazaga, SF; Angulo-Barturen, I; Waterson, D; Burrows, JN; Matthews, D; Charman, SA; Phillips, MA; Rathod, PK Tetrahydro-2-naphthyl and 2-Indanyl Triazolopyrimidines Targeting Plasmodium falciparum Dihydroorotate Dehydrogenase Display Potent and Selective Antimalarial Activity. J Med Chem59:5416-31 (2016) [PubMed] Article
Kokkonda, S; Deng, X; White, KL; Coteron, JM; Marco, M; de Las Heras, L; White, J; El Mazouni, F; Tomchick, DR; Manjalanagara, K; Rudra, KR; Chen, G; Morizzi, J; Ryan, E; Kaminsky, W; Leroy, D; Martínez-Martínez, MS; Jimenez-Diaz, MB; Bazaga, SF; Angulo-Barturen, I; Waterson, D; Burrows, JN; Matthews, D; Charman, SA; Phillips, MA; Rathod, PK Tetrahydro-2-naphthyl and 2-Indanyl Triazolopyrimidines Targeting Plasmodium falciparum Dihydroorotate Dehydrogenase Display Potent and Selective Antimalarial Activity. J Med Chem59:5416-31 (2016) [PubMed] Article