

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86244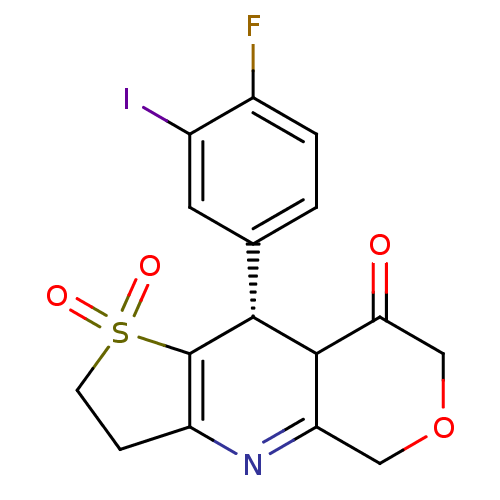 (A-312110) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86250 (CAS_60559-98-0 | NSC_43345 | P1075) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86250 (CAS_60559-98-0 | NSC_43345 | P1075) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Br J Pharmacol 143: 81-90 (2004) Article DOI: 10.1038/sj.bjp.0705908 BindingDB Entry DOI: 10.7270/Q2QR4VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86247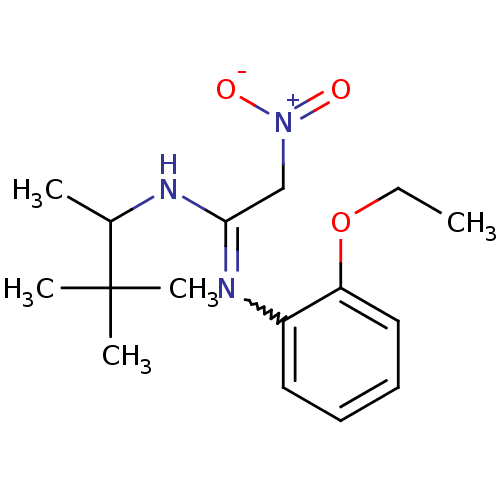 (BAY X 9228 | CAS_3038059 | NSC_3038059) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86250 (CAS_60559-98-0 | NSC_43345 | P1075) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86247 (BAY X 9228 | CAS_3038059 | NSC_3038059) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M41L/D67N/L210W/T215Y mutant by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M41L/D67N/L210W/T215Y mutant by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86245 (CAS_85371-64-8 | NSC_4826 | PINACIDIL) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478973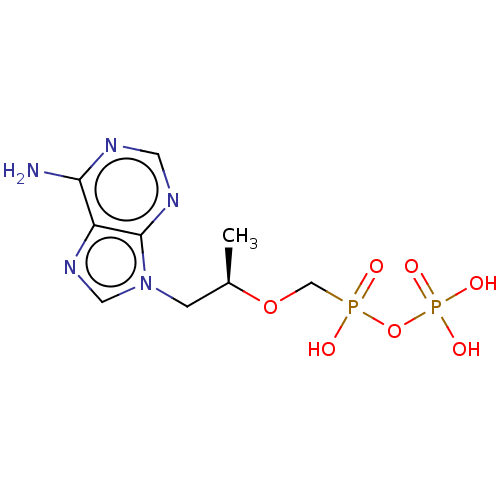 (CHEMBL1162283) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M41L/D67N/L210W/T215Y mutant by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478974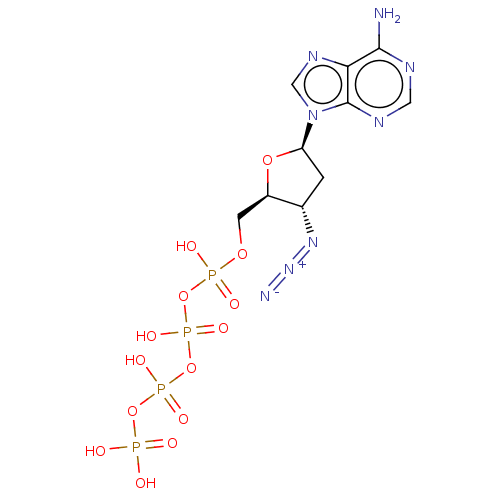 (CHEMBL1162284) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M41L/D67N/L210W/T215Y mutant by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86251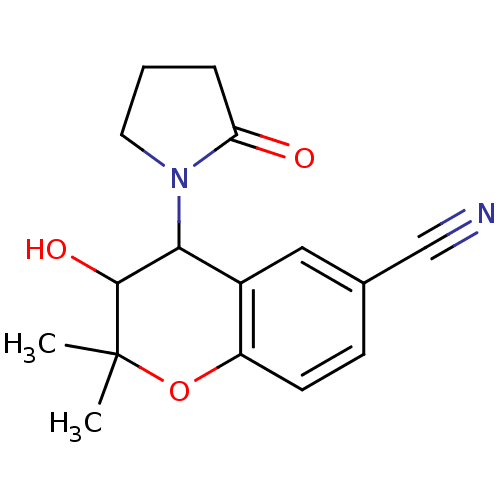 (CAS_94535-50-9 | CROMAKALIM (-) | NSC_93504) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478973 (CHEMBL1162283) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478974 (CHEMBL1162284) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by steady state nucleotide incorporation assay | Antimicrob Agents Chemother 51: 2911-9 (2007) Article DOI: 10.1128/aac.00314-07 BindingDB Entry DOI: 10.7270/Q2CV4MJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86245 (CAS_85371-64-8 | NSC_4826 | PINACIDIL) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86249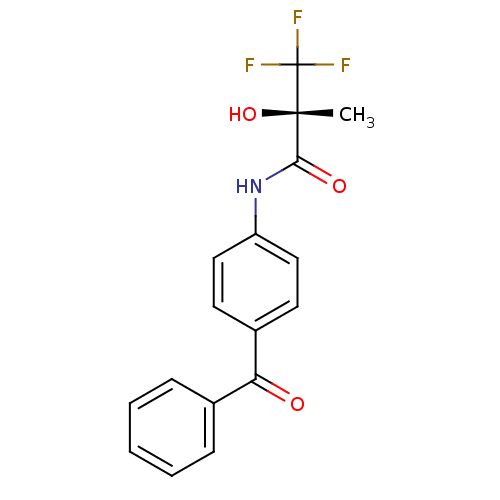 (ZD 6169 | ZD-6169) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86251 (CAS_94535-50-9 | CROMAKALIM (-) | NSC_93504) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86249 (ZD 6169 | ZD-6169) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86246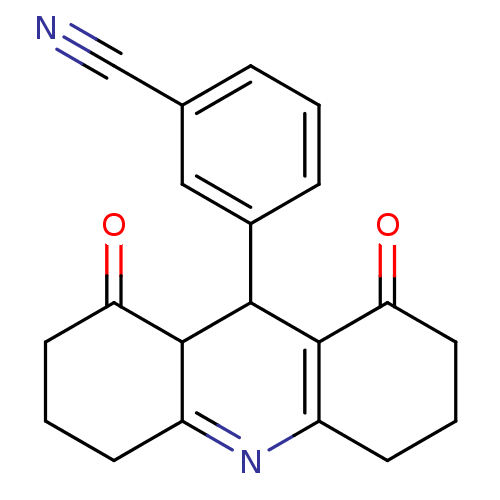 (zm244085) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 671 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86246 (zm244085) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86248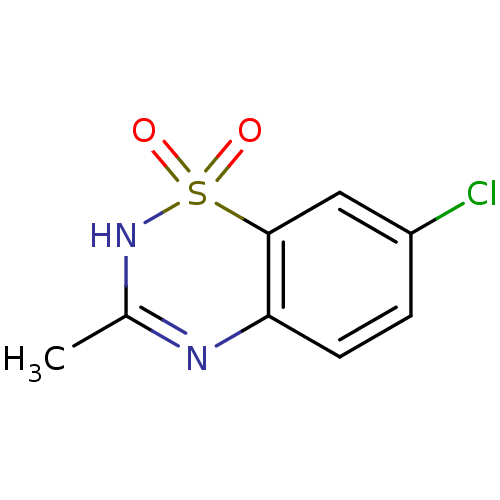 (CAS_364-98-7 | NSC_3019 | diazoxide) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-activated potassium channel subunit beta (GUINEA PIG) | BDBM86248 (CAS_364-98-7 | NSC_3019 | diazoxide) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 64: 143-53 (2003) Article DOI: 10.1124/mol.64.1.143 BindingDB Entry DOI: 10.7270/Q22B8WMJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518833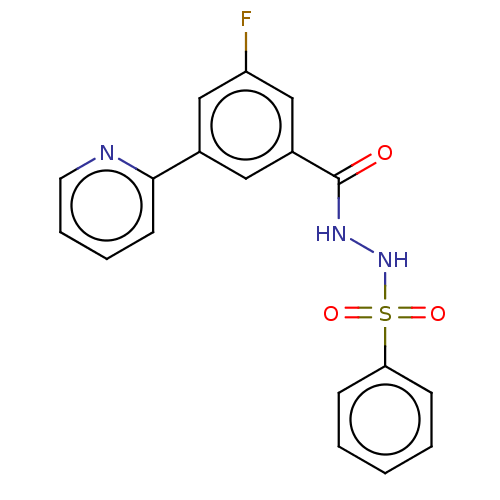 (CHEMBL4528993 | US10829446, Compound 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527224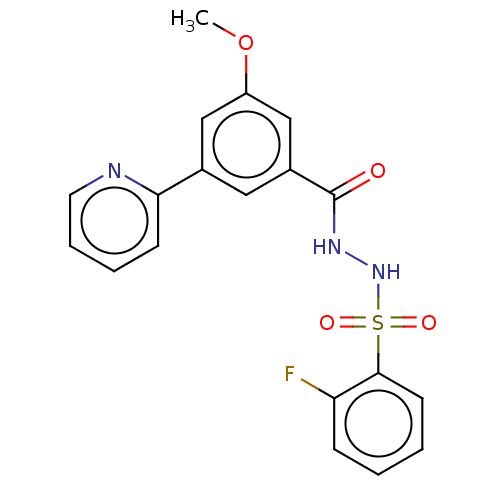 (CHEMBL4468833 | US10829446, Compound 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527244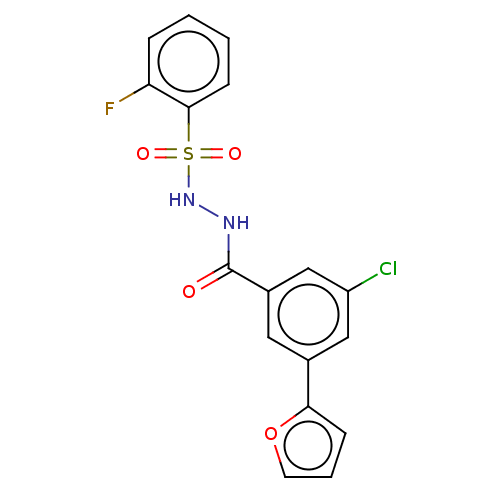 (CHEMBL4437798 | US10829446, Compound 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527381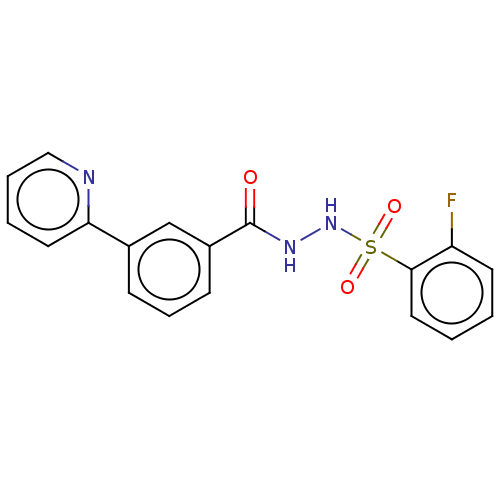 (CHEMBL4466823 | US10829446, Compound 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527355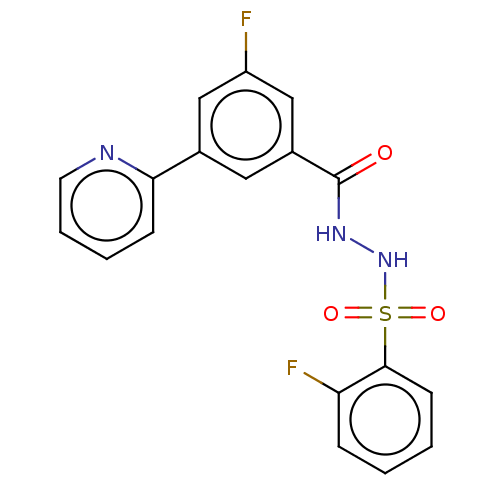 (CHEMBL4456585 | US10829446, Compound 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527250 (CHEMBL4475883 | US10829446, Compound 67) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518832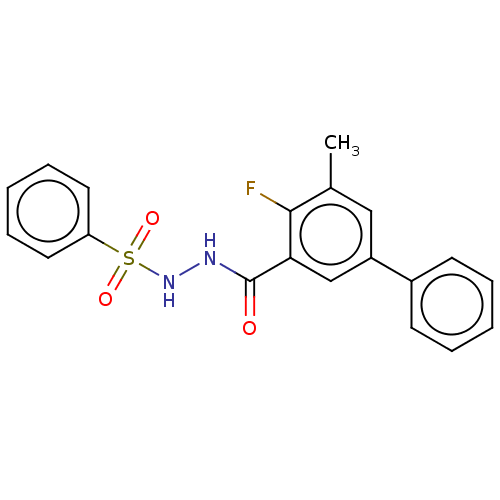 (CHEMBL4455897 | US10829446, Compound 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527238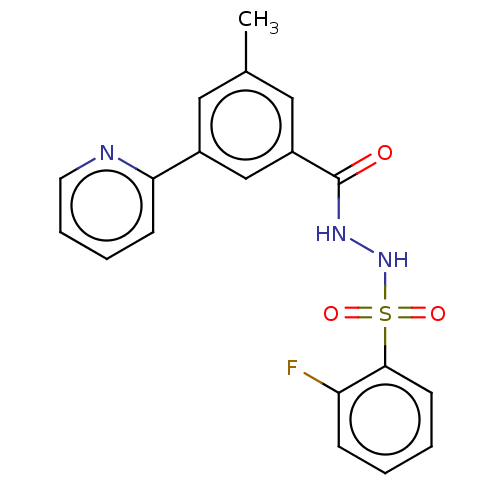 (CHEMBL4539384 | US10829446, Compound 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527328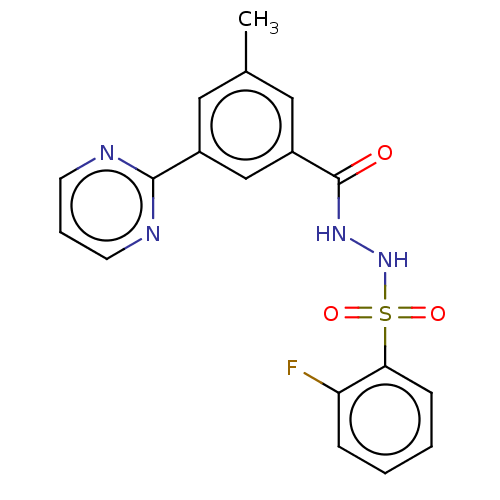 (CHEMBL4441439 | US10829446, Compound 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527245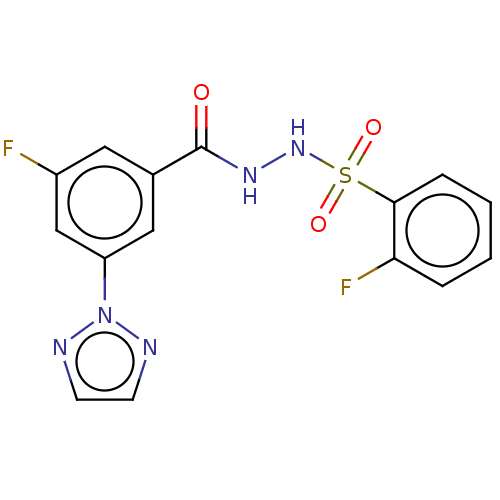 (CHEMBL4521125 | US10829446, Compound 89) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527225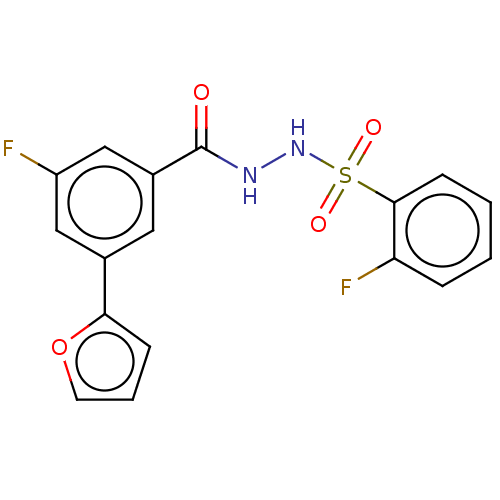 (CHEMBL4565790 | US10829446, Compound 101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518831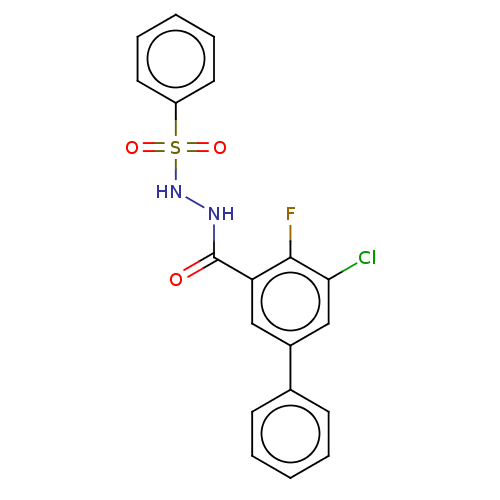 (CHEMBL4464835 | US10829446, Compound 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527243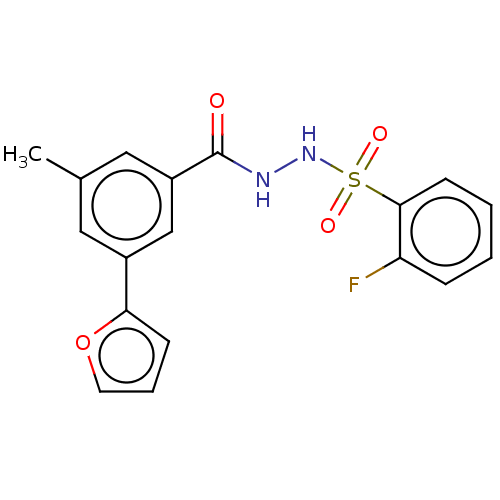 (CHEMBL4458272 | US10829446, Compound 153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239647 (CHEMBL4092595) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527263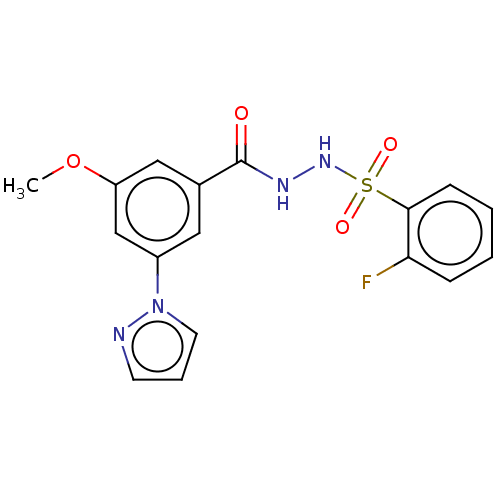 (CHEMBL4465716 | US10829446, Compound 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527246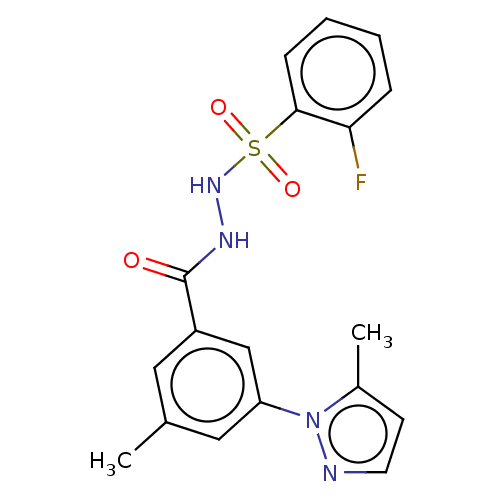 (CHEMBL4445473 | US10829446, Compound 84) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527235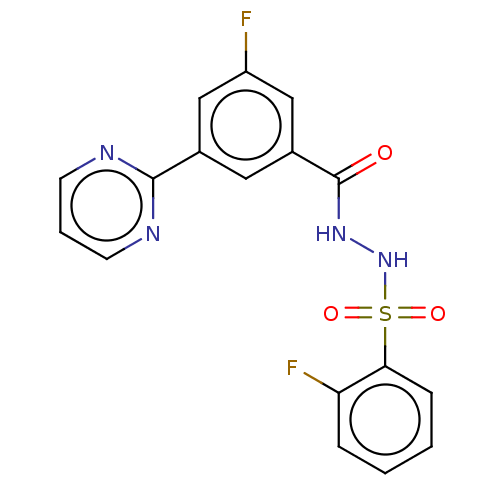 (CHEMBL4516984 | US10829446, Compound 52) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527240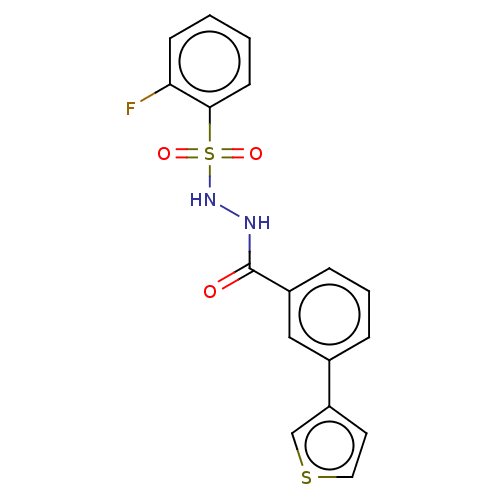 (CHEMBL4541948 | US10829446, Compound 124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527232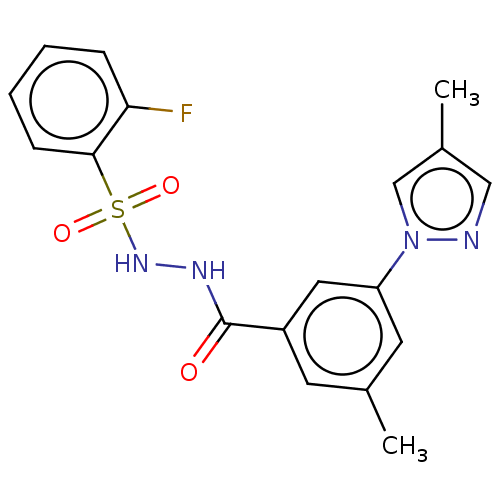 (CHEMBL4436877 | US10829446, Compound 78) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527366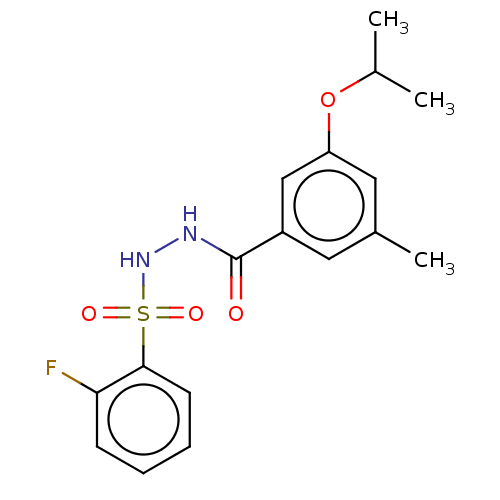 (CHEMBL4449163 | US10829446, Compound 139) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527382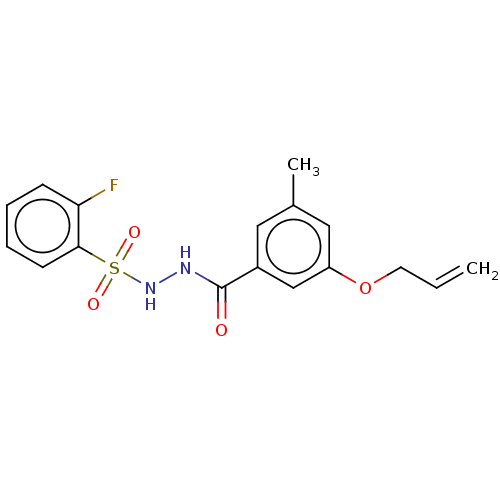 (CHEMBL4518462 | US10829446, Compound 145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527313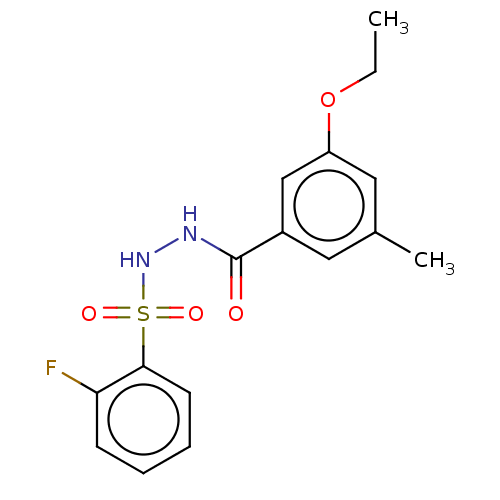 (CHEMBL4446917 | US10829446, Compound 137) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Rattus norvegicus (rat)) | BDBM50018011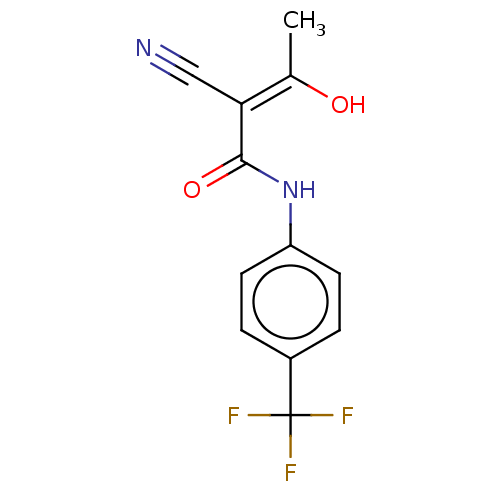 (Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527277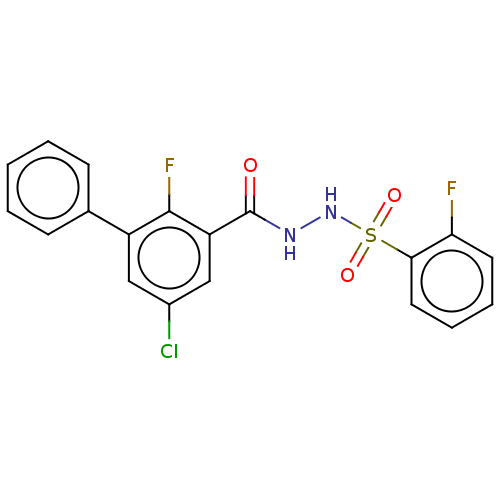 (CHEMBL4593500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518867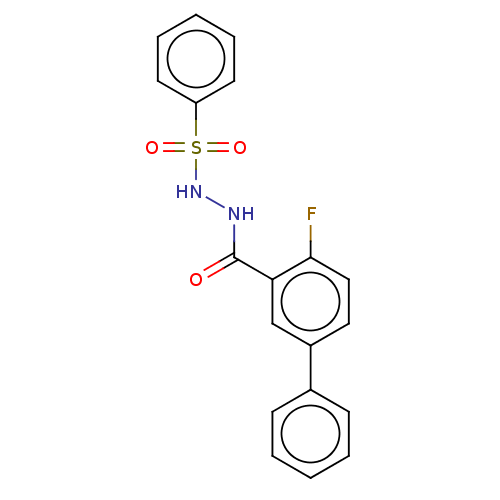 (CHEMBL4460509 | US10829446, Compound 157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527302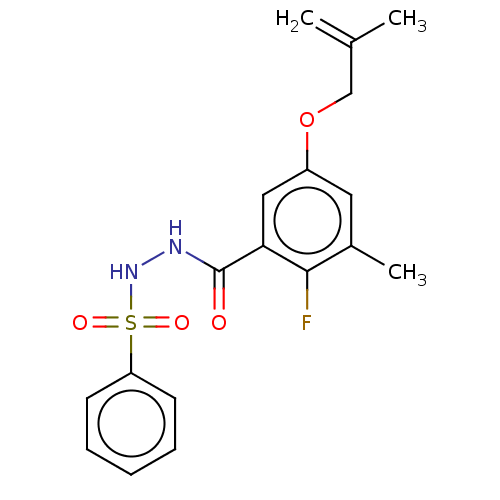 (CHEMBL4573736 | US10829446, Compound 146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1290 total ) | Next | Last >> |