| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Ligand | BDBM185149 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2087564 (CHEMBL4768827) |
|---|
| IC50 | 1.3±n/a nM |
|---|
| Citation |  Jeong, P; Moon, Y; Lee, JH; Lee, SD; Park, J; Lee, J; Kim, J; Lee, HJ; Kim, NY; Choi, J; Heo, JD; Shin, JE; Park, HW; Kim, YG; Han, SY; Kim, YC Discovery of orally active indirubin-3'-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur J Med Chem195:0 (2020) [PubMed] Article Jeong, P; Moon, Y; Lee, JH; Lee, SD; Park, J; Lee, J; Kim, J; Lee, HJ; Kim, NY; Choi, J; Heo, JD; Shin, JE; Park, HW; Kim, YG; Han, SY; Kim, YC Discovery of orally active indirubin-3'-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur J Med Chem195:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Name: | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Synonyms: | CD135 | CD_antigen: CD135 | FL cytokine receptor | FLK-2 | FLK2 | FLT-3 | FLT3 | FLT3_HUMAN | Fetal liver kinase-2 | Fms-like tyrosine kinase 3 | Fms-like tyrosine kinase 3 (Flt-3) | Fms-related tyrosine kinase 3 | STK-1 | STK1 | Stem cell tyrosine kinase 1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 112888.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P36888 |
|---|
| Residue: | 993 |
|---|
| Sequence: | MPALARDGGQLPLLVVFSAMIFGTITNQDLPVIKCVLINHKNNDSSVGKSSSYPMVSESP
EDLGCALRPQSSGTVYEAAAVEVDVSASITLQVLVDAPGNISCLWVFKHSSLNCQPHFDL
QNRGVVSMVILKMTETQAGEYLLFIQSEATNYTILFTVSIRNTLLYTLRRPYFRKMENQD
ALVCISESVPEPIVEWVLCDSQGESCKEESPAVVKKEEKVLHELFGTDIRCCARNELGRE
CTRLFTIDLNQTPQTTLPQLFLKVGEPLWIRCKAVHVNHGFGLTWELENKALEEGNYFEM
STYSTNRTMIRILFAFVSSVARNDTGYYTCSSSKHPSQSALVTIVEKGFINATNSSEDYE
IDQYEEFCFSVRFKAYPQIRCTWTFSRKSFPCEQKGLDNGYSISKFCNHKHQPGEYIFHA
ENDDAQFTKMFTLNIRRKPQVLAEASASQASCFSDGYPLPSWTWKKCSDKSPNCTEEITE
GVWNRKANRKVFGQWVSSSTLNMSEAIKGFLVKCCAYNSLGTSCETILLNSPGPFPFIQD
NISFYATIGVCLLFIVVLTLLICHKYKKQFRYESQLQMVQVTGSSDNEYFYVDFREYEYD
LKWEFPRENLEFGKVLGSGAFGKVMNATAYGISKTGVSIQVAVKMLKEKADSSEREALMS
ELKMMTQLGSHENIVNLLGACTLSGPIYLIFEYCCYGDLLNYLRSKREKFHRTWTEIFKE
HNFSFYPTFQSHPNSSMPGSREVQIHPDSDQISGLHGNSFHSEDEIEYENQKRLEEEEDL
NVLTFEDLLCFAYQVAKGMEFLEFKSCVHRDLAARNVLVTHGKVVKICDFGLARDIMSDS
NYVVRGNARLPVKWMAPESLFEGIYTIKSDVWSYGILLWEIFSLGVNPYPGIPVDANFYK
LIQNGFKMDQPFYATEEIYIIMQSCWAFDSRKRPSFPNLTSFLGCQLADAEEAMYQNVDG
RVSECPHTYQNRRPFSREMDLGLLSPQAQVEDS
|
|
|
|---|
| BDBM185149 |
|---|
| n/a |
|---|
| Name | BDBM185149 |
|---|
| Synonyms: | 1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol-1-yl]quinolin-8-yl]piperidin-4-amine | Crenolanib | US11542261, Compound crenolanib |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H29N5O2 |
|---|
| Mol. Mass. | 443.5408 |
|---|
| SMILES | CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 |
|---|
| Structure | 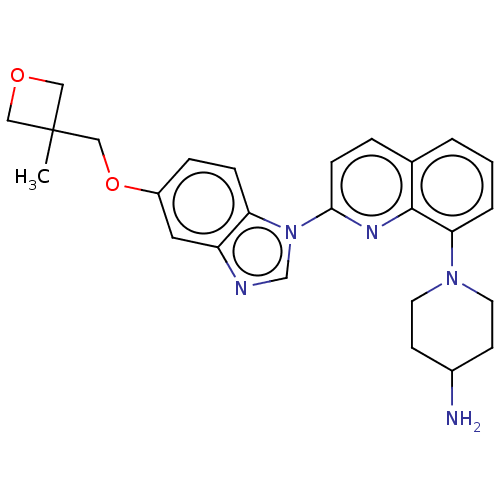
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jeong, P; Moon, Y; Lee, JH; Lee, SD; Park, J; Lee, J; Kim, J; Lee, HJ; Kim, NY; Choi, J; Heo, JD; Shin, JE; Park, HW; Kim, YG; Han, SY; Kim, YC Discovery of orally active indirubin-3'-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur J Med Chem195:0 (2020) [PubMed] Article
Jeong, P; Moon, Y; Lee, JH; Lee, SD; Park, J; Lee, J; Kim, J; Lee, HJ; Kim, NY; Choi, J; Heo, JD; Shin, JE; Park, HW; Kim, YG; Han, SY; Kim, YC Discovery of orally active indirubin-3'-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur J Med Chem195:0 (2020) [PubMed] Article