 Found 3377 hits with Last Name = 'lee' and Initial = 'jh'
Found 3377 hits with Last Name = 'lee' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063266
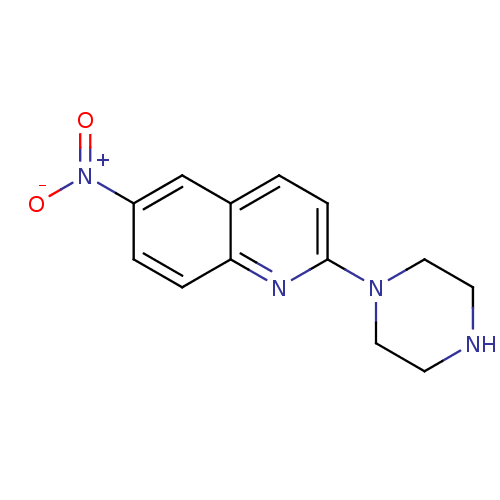
(6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...)Show InChI InChI=1S/C13H14N4O2/c18-17(19)11-2-3-12-10(9-11)1-4-13(15-12)16-7-5-14-6-8-16/h1-4,9,14H,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50180197
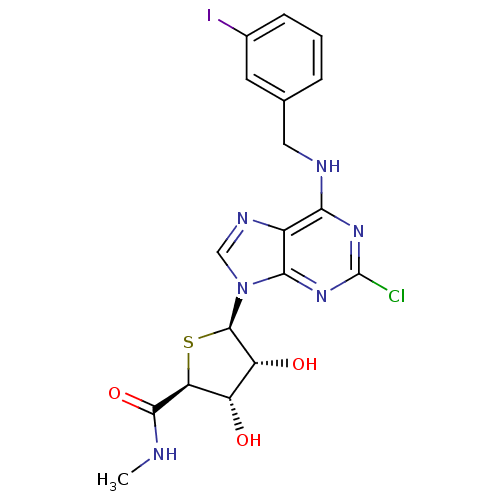
((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
J Med Chem 55: 342-56 (2012)
Article DOI: 10.1021/jm201229j
BindingDB Entry DOI: 10.7270/Q2VQ334S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50180197

((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed CHO cells after 60 mins by gamma counting |
Bioorg Med Chem 18: 7015-21 (2010)
Article DOI: 10.1016/j.bmc.2010.08.018
BindingDB Entry DOI: 10.7270/Q21N81CV |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248937
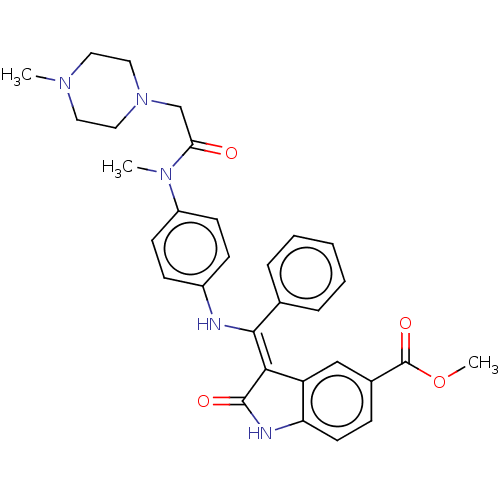
(CHEMBL4062168 | US10981896, Compound 15)Show SMILES COC(=O)c1ccc2NC(=O)\C(=C(/Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-19-22(31(39)40-3)9-14-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
MELK and its substrate, Bcl-G were both recombinantly expressed and purified for use in screening assays (See Methods). 752 compounds from an in-hous... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061352
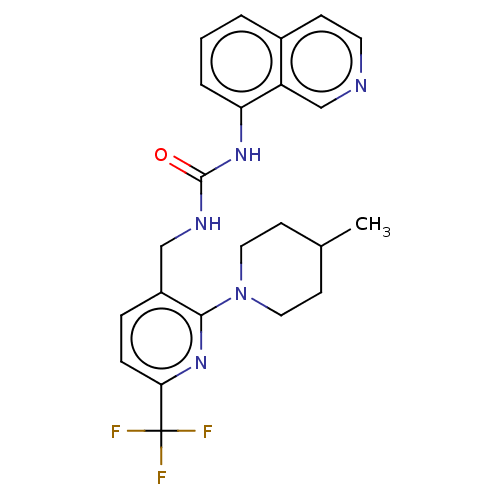
(CHEMBL3393836)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2ccncc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)13-28-22(32)29-19-4-2-3-16-7-10-27-14-18(16)19/h2-7,10,14-15H,8-9,11-13H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208769
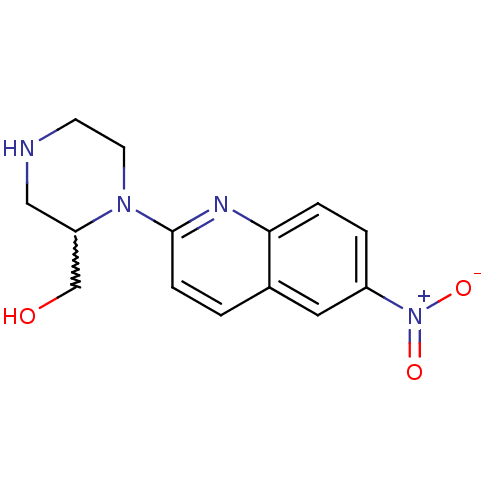
(2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...)Show SMILES OCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:2.1| Show InChI InChI=1S/C14H16N4O3/c19-9-12-8-15-5-6-17(12)14-4-1-10-7-11(18(20)21)2-3-13(10)16-14/h1-4,7,12,15,19H,5-6,8-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208770

(2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...)Show SMILES CCOCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:4.3| Show InChI InChI=1S/C16H20N4O3/c1-2-23-11-14-10-17-7-8-19(14)16-6-3-12-9-13(20(21)22)4-5-15(12)18-16/h3-6,9,14,17H,2,7-8,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248946
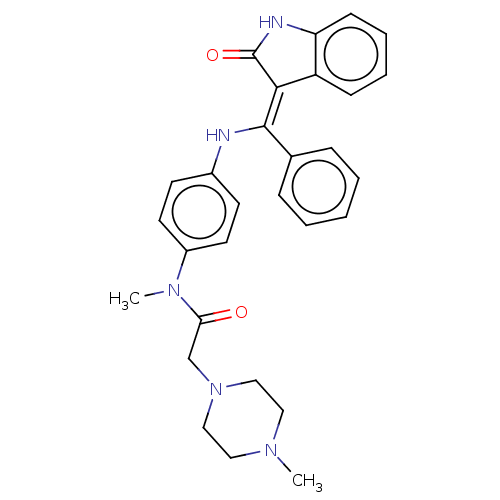
(CHEMBL1908392 | US10981896, Compound 16)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccccc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H31N5O2/c1-32-16-18-34(19-17-32)20-26(35)33(2)23-14-12-22(13-15-23)30-28(21-8-4-3-5-9-21)27-24-10-6-7-11-25(24)31-29(27)36/h3-15,30H,16-20H2,1-2H3,(H,31,36)/b28-27- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
MELK and its substrate, Bcl-G were both recombinantly expressed and purified for use in screening assays (See Methods). 752 compounds from an in-hous... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385670
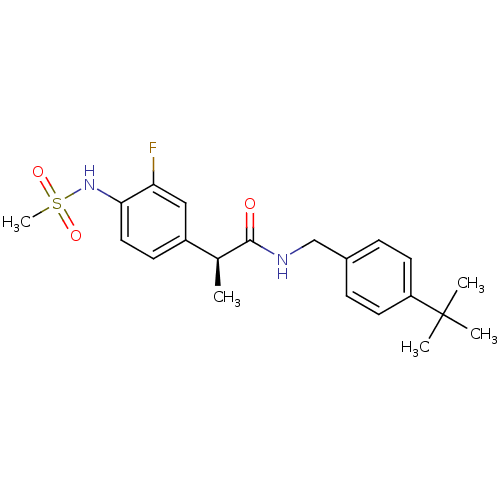
(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208771

(2-[(2-methoxymethyl)piperazin-1-yl]-6-nitroquinoli...)Show SMILES COCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:3.2| Show InChI InChI=1S/C15H18N4O3/c1-22-10-13-9-16-6-7-18(13)15-5-2-11-8-12(19(20)21)3-4-14(11)17-15/h2-5,8,13,16H,6-7,9-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248938

(CHEMBL4089284 | US10981896, Compound 21)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccc(cc23)C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C30H31N5O4/c1-33-14-16-35(17-15-33)19-26(36)34(2)23-11-9-22(10-12-23)31-28(20-6-4-3-5-7-20)27-24-18-21(30(38)39)8-13-25(24)32-29(27)37/h3-13,18,31H,14-17,19H2,1-2H3,(H,32,37)(H,38,39)/b28-27- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
MELK and its substrate, Bcl-G were both recombinantly expressed and purified for use in screening assays (See Methods). 752 compounds from an in-hous... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061319
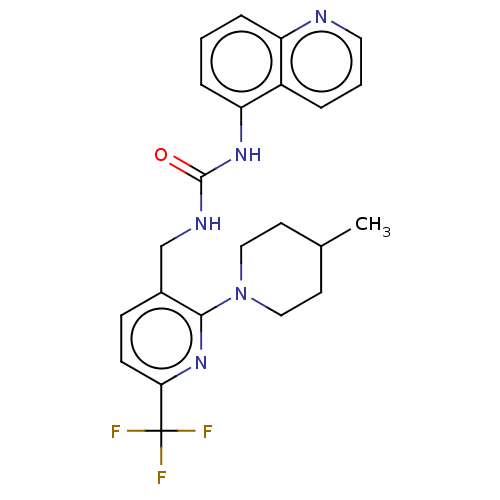
(CHEMBL3393838)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2ncccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-9-12-31(13-10-15)21-16(7-8-20(30-21)23(24,25)26)14-28-22(32)29-19-6-2-5-18-17(19)4-3-11-27-18/h2-8,11,15H,9-10,12-14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272598
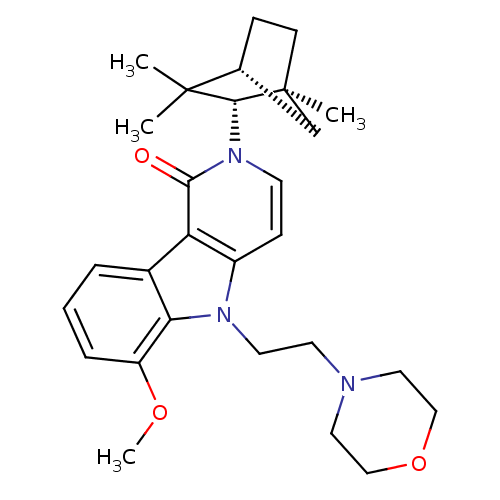
(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
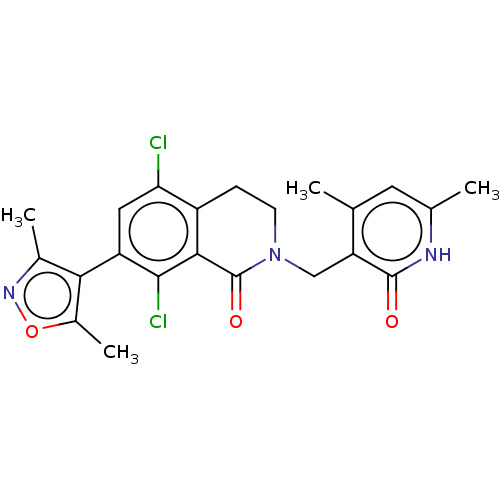
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02112
BindingDB Entry DOI: 10.7270/Q23J3HPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50293031
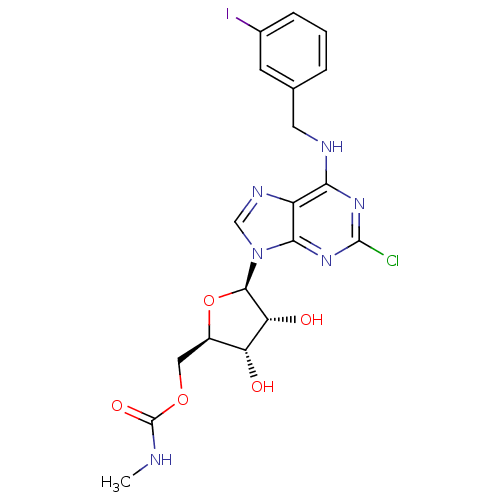
(2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...)Show SMILES CNC(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H20ClIN6O5/c1-22-19(30)31-7-11-13(28)14(29)17(32-11)27-8-24-12-15(25-18(20)26-16(12)27)23-6-9-3-2-4-10(21)5-9/h2-5,8,11,13-14,17,28-29H,6-7H2,1H3,(H,22,30)(H,23,25,26)/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
J Med Chem 55: 342-56 (2012)
Article DOI: 10.1021/jm201229j
BindingDB Entry DOI: 10.7270/Q2VQ334S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50214981
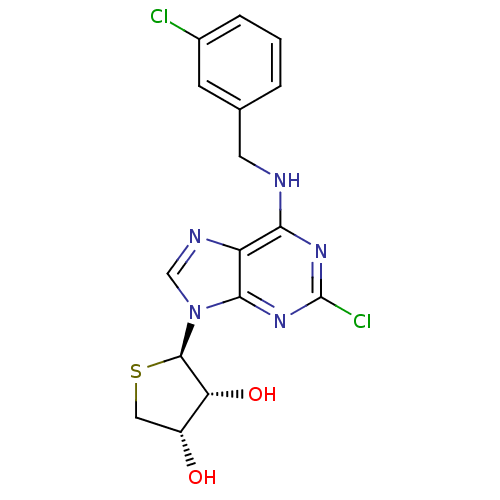
((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15Cl2N5O2S/c17-9-3-1-2-8(4-9)5-19-13-11-14(22-16(18)21-13)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells at 10 uM |
ACS Med Chem Lett 9: 516-520 (2010)
Article DOI: 10.1021/ml1001823
BindingDB Entry DOI: 10.7270/Q24T6KCC |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50248946

(CHEMBL1908392 | US10981896, Compound 16)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccccc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H31N5O2/c1-32-16-18-34(19-17-32)20-26(35)33(2)23-14-12-22(13-15-23)30-28(21-8-4-3-5-9-21)27-24-10-6-7-11-25(24)31-29(27)36/h3-15,30H,16-20H2,1-2H3,(H,31,36)/b28-27- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061302

(CHEMBL3393840)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cncc2ccccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-10-31(11-9-15)21-17(6-7-20(30-21)23(24,25)26)13-28-22(32)29-19-14-27-12-16-4-2-3-5-18(16)19/h2-7,12,14-15H,8-11,13H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50562994
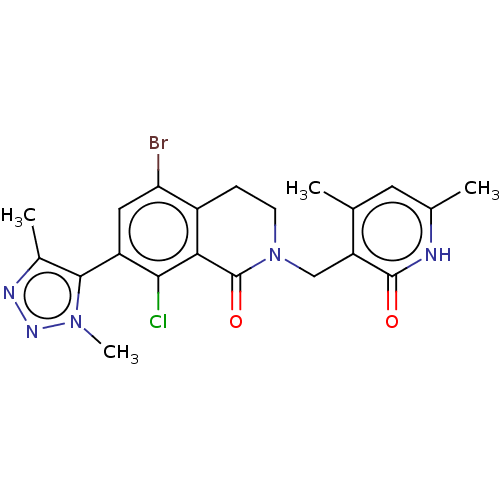
(CHEMBL4740532)Show SMILES Cc1nnn(C)c1-c1cc(Br)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(30.21,-10.38,;29.89,-11.89,;30.92,-13.04,;30.16,-14.37,;28.65,-14.05,;27.51,-15.09,;28.48,-12.52,;27.14,-11.76,;25.8,-12.53,;24.47,-11.76,;23.14,-12.52,;24.48,-10.22,;23.14,-9.45,;23.14,-7.92,;24.48,-7.16,;24.48,-5.61,;23.14,-4.84,;23.14,-3.29,;24.47,-2.52,;21.8,-2.53,;20.47,-3.3,;19.13,-2.53,;20.47,-4.84,;21.8,-5.62,;21.8,-7.16,;25.82,-7.91,;27.15,-7.14,;25.81,-9.45,;27.14,-10.22,;28.46,-9.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02112
BindingDB Entry DOI: 10.7270/Q23J3HPC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50580163
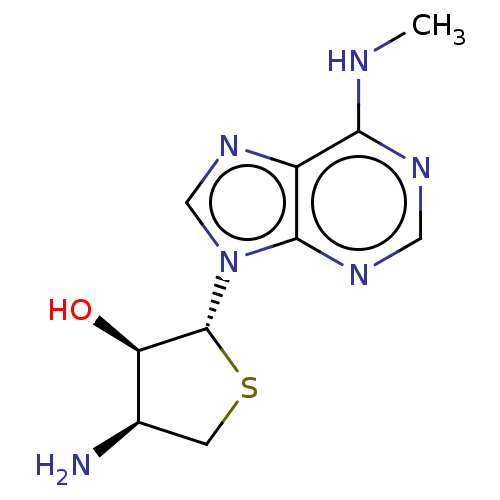
(CHEMBL5075606)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1SC[C@@H](N)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane incubated for 60 mins by gamma counter method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00239
BindingDB Entry DOI: 10.7270/Q2PC367C |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709

(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type EZH2 Y641N mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02112
BindingDB Entry DOI: 10.7270/Q23J3HPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50385670

(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr by ... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50214974
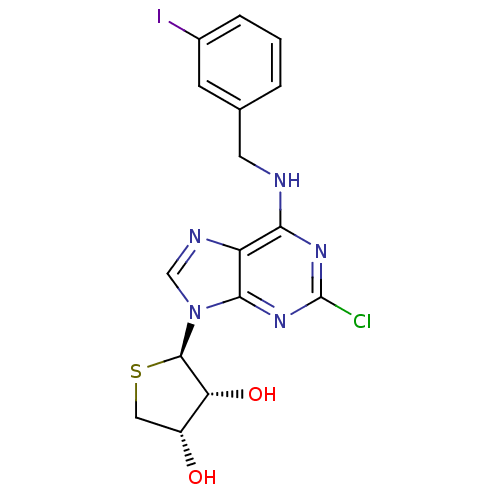
((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed CHO cells after 60 mins by gamma counting |
Bioorg Med Chem 18: 7015-21 (2010)
Article DOI: 10.1016/j.bmc.2010.08.018
BindingDB Entry DOI: 10.7270/Q21N81CV |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50248946

(CHEMBL1908392 | US10981896, Compound 16)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccccc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H31N5O2/c1-32-16-18-34(19-17-32)20-26(35)33(2)23-14-12-22(13-15-23)30-28(21-8-4-3-5-9-21)27-24-10-6-7-11-25(24)31-29(27)36/h3-15,30H,16-20H2,1-2H3,(H,31,36)/b28-27- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50248938

(CHEMBL4089284 | US10981896, Compound 21)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccc(cc23)C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C30H31N5O4/c1-33-14-16-35(17-15-33)19-26(36)34(2)23-11-9-22(10-12-23)31-28(20-6-4-3-5-7-20)27-24-18-21(30(38)39)8-13-25(24)32-29(27)37/h3-13,18,31H,14-17,19H2,1-2H3,(H,32,37)(H,38,39)/b28-27- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385656
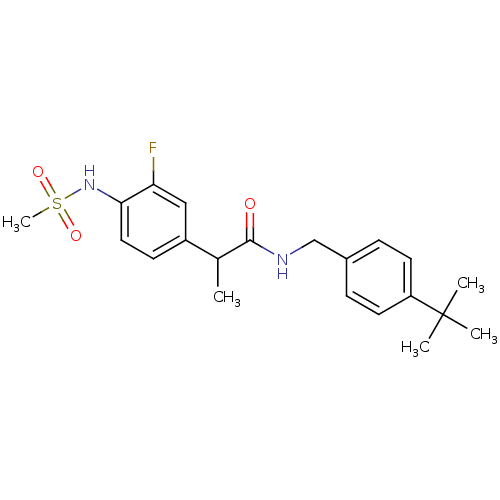
(CHEMBL2042264)Show SMILES CC(C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272567
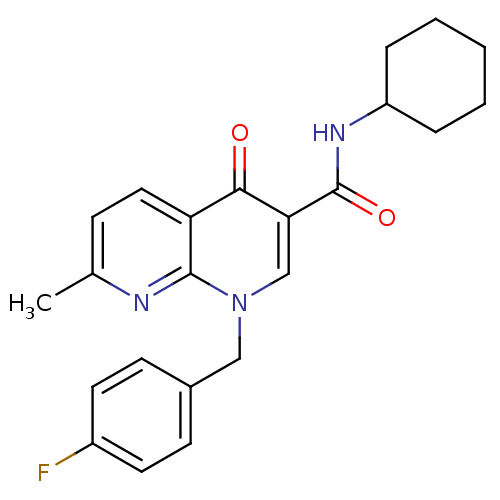
(1-(4-fluorobenzyl)-N-cyclohexyl-7-methyl-4-oxo-1,4...)Show SMILES Cc1ccc2c(n1)n(Cc1ccc(F)cc1)cc(C(=O)NC1CCCCC1)c2=O Show InChI InChI=1S/C23H24FN3O2/c1-15-7-12-19-21(28)20(23(29)26-18-5-3-2-4-6-18)14-27(22(19)25-15)13-16-8-10-17(24)11-9-16/h7-12,14,18H,2-6,13H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208772
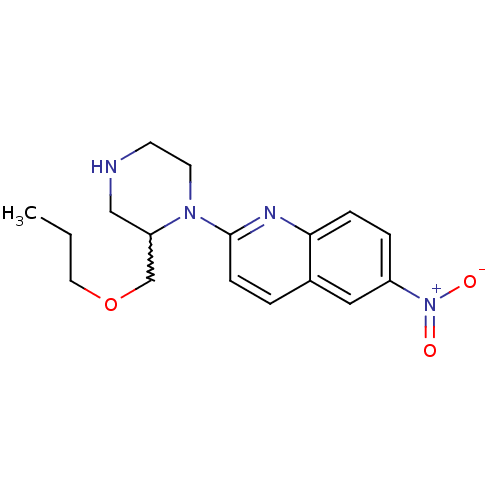
(6-nitro-2-(2-propoxymethylpiperazin-1-yl)quinoline...)Show SMILES CCCOCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:5.4| Show InChI InChI=1S/C17H22N4O3/c1-2-9-24-12-15-11-18-7-8-20(15)17-6-3-13-10-14(21(22)23)4-5-16(13)19-17/h3-6,10,15,18H,2,7-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50248937

(CHEMBL4062168 | US10981896, Compound 15)Show SMILES COC(=O)c1ccc2NC(=O)\C(=C(/Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-19-22(31(39)40-3)9-14-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50562994

(CHEMBL4740532)Show SMILES Cc1nnn(C)c1-c1cc(Br)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(30.21,-10.38,;29.89,-11.89,;30.92,-13.04,;30.16,-14.37,;28.65,-14.05,;27.51,-15.09,;28.48,-12.52,;27.14,-11.76,;25.8,-12.53,;24.47,-11.76,;23.14,-12.52,;24.48,-10.22,;23.14,-9.45,;23.14,-7.92,;24.48,-7.16,;24.48,-5.61,;23.14,-4.84,;23.14,-3.29,;24.47,-2.52,;21.8,-2.53,;20.47,-3.3,;19.13,-2.53,;20.47,-4.84,;21.8,-5.62,;21.8,-7.16,;25.82,-7.91,;27.15,-7.14,;25.81,-9.45,;27.14,-10.22,;28.46,-9.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type EZH2 Y641N mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02112
BindingDB Entry DOI: 10.7270/Q23J3HPC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061359
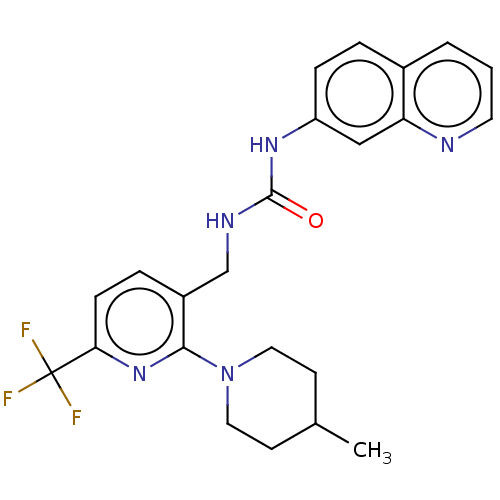
(CHEMBL3393826)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1ccc2cccnc2c1)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-7-20(30-21)23(24,25)26)14-28-22(32)29-18-6-4-16-3-2-10-27-19(16)13-18/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50339076

((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...)Show SMILES CCCCC#Cc1nc(N)c2ncn([C@@H]3SC[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C15H19N5O2S/c1-2-3-4-5-6-10-18-13(16)11-14(19-10)20(8-17-11)15-12(22)9(21)7-23-15/h8-9,12,15,21-22H,2-4,7H2,1H3,(H2,16,18,19)/t9-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human adenosine A2A receptor in HEK293 cells |
ACS Med Chem Lett 9: 516-520 (2010)
Article DOI: 10.1021/ml1001823
BindingDB Entry DOI: 10.7270/Q24T6KCC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50339076

((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...)Show SMILES CCCCC#Cc1nc(N)c2ncn([C@@H]3SC[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C15H19N5O2S/c1-2-3-4-5-6-10-18-13(16)11-14(19-10)20(8-17-11)15-12(22)9(21)7-23-15/h8-9,12,15,21-22H,2-4,7H2,1H3,(H2,16,18,19)/t9-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting |
J Med Chem 55: 342-56 (2012)
Article DOI: 10.1021/jm201229j
BindingDB Entry DOI: 10.7270/Q2VQ334S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061357
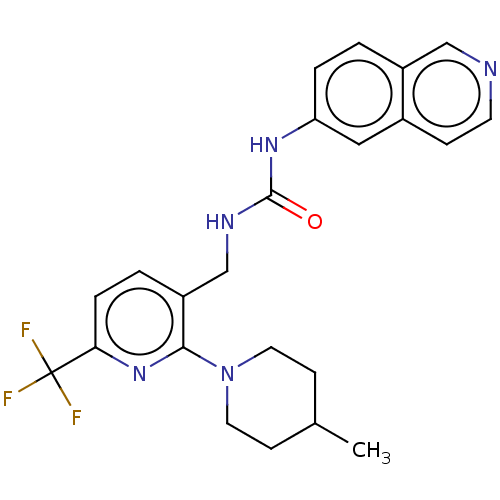
(CHEMBL3393828)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1ccc2cnccc2c1)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-7-10-31(11-8-15)21-18(3-5-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-17-13-27-9-6-16(17)12-19/h2-6,9,12-13,15H,7-8,10-11,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50385671
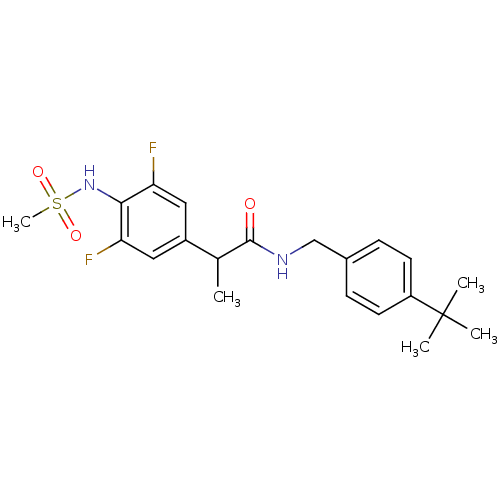
(CHEMBL2042384)Show SMILES CC(C(=O)NCc1ccc(cc1)C(C)(C)C)c1cc(F)c(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H26F2N2O3S/c1-13(15-10-17(22)19(18(23)11-15)25-29(5,27)28)20(26)24-12-14-6-8-16(9-7-14)21(2,3)4/h6-11,13,25H,12H2,1-5H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr by ... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
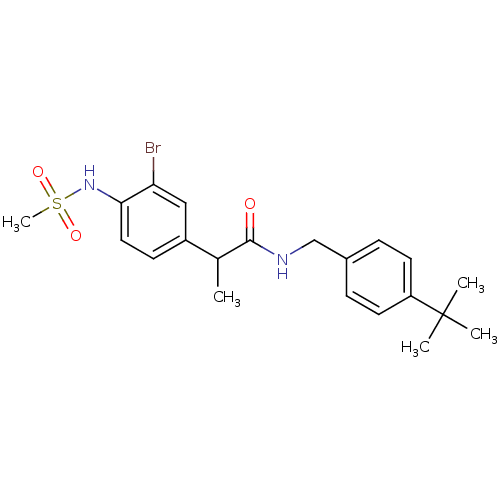
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50385658
(CHEMBL2042266)Show SMILES CC(C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Br)c1 Show InChI InChI=1S/C21H27BrN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50385656

(CHEMBL2042264)Show SMILES CC(C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr by ... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
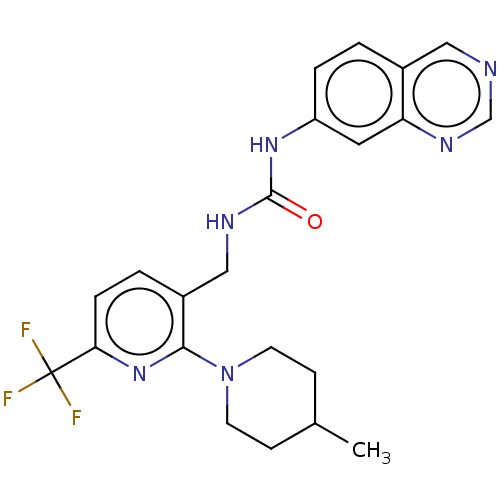
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061354
(CHEMBL3393833)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1ccc2cncnc2c1)C(F)(F)F Show InChI InChI=1S/C22H23F3N6O/c1-14-6-8-31(9-7-14)20-16(3-5-19(30-20)22(23,24)25)12-27-21(32)29-17-4-2-15-11-26-13-28-18(15)10-17/h2-5,10-11,13-14H,6-9,12H2,1H3,(H2,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385670

(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in pH-induced intracellular 45Ca2+ uptake after 1 hr by fluor... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
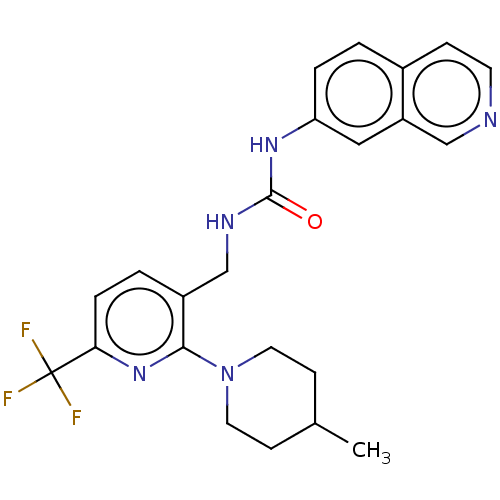
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061358
(CHEMBL3393827)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1ccc2ccncc2c1)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-7-10-31(11-8-15)21-17(3-5-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-16-6-9-27-13-18(16)12-19/h2-6,9,12-13,15H,7-8,10-11,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50248938

(CHEMBL4089284 | US10981896, Compound 21)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccc(cc23)C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C30H31N5O4/c1-33-14-16-35(17-15-33)19-26(36)34(2)23-11-9-22(10-12-23)31-28(20-6-4-3-5-7-20)27-24-18-21(30(38)39)8-13-25(24)32-29(27)37/h3-13,18,31H,14-17,19H2,1-2H3,(H,32,37)(H,38,39)/b28-27- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM30130
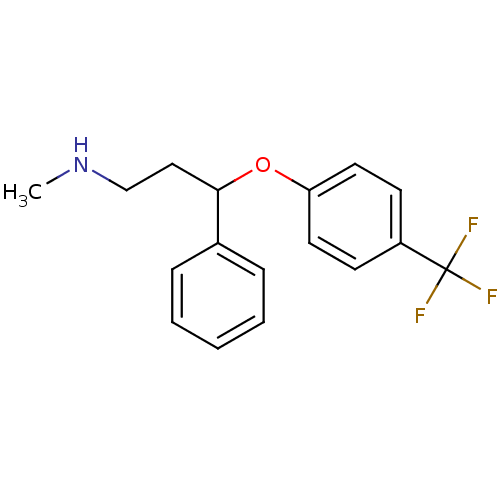
(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061355
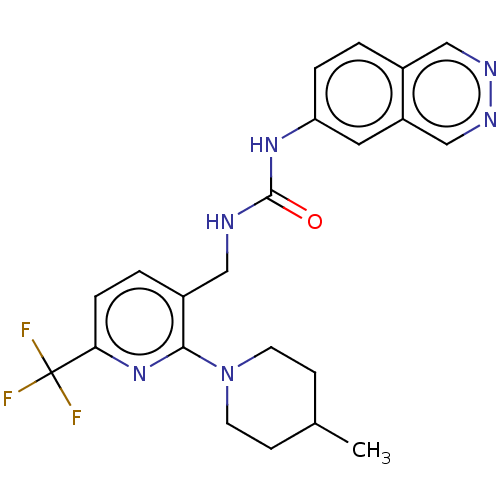
(CHEMBL3393832)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1ccc2cnncc2c1)C(F)(F)F Show InChI InChI=1S/C22H23F3N6O/c1-14-6-8-31(9-7-14)20-16(3-5-19(30-20)22(23,24)25)11-26-21(32)29-18-4-2-15-12-27-28-13-17(15)10-18/h2-5,10,12-14H,6-9,11H2,1H3,(H2,26,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50248937

(CHEMBL4062168 | US10981896, Compound 15)Show SMILES COC(=O)c1ccc2NC(=O)\C(=C(/Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-19-22(31(39)40-3)9-14-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
Candidates for inhibitor selectivity characterization were chosen based on an initial single-timepoint commercial kinome profiling screen performed w... |
US Patent US10981896 (2021)
BindingDB Entry DOI: 10.7270/Q24X5BWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20321
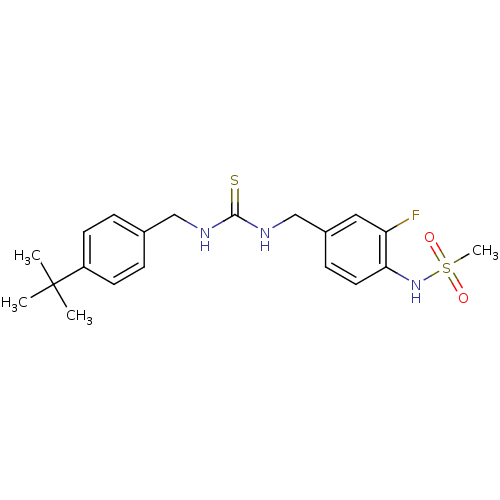
(3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NCc2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C20H26FN3O2S2/c1-20(2,3)16-8-5-14(6-9-16)12-22-19(27)23-13-15-7-10-18(17(21)11-15)24-28(4,25)26/h5-11,24H,12-13H2,1-4H3,(H2,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr by ... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061356

(CHEMBL3393830)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cnc2ccccc2c1)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-10-31(11-9-15)21-17(6-7-20(30-21)23(24,25)26)13-28-22(32)29-18-12-16-4-2-3-5-19(16)27-14-18/h2-7,12,14-15H,8-11,13H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data