| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Melanocortin receptor 4 |
|---|
| Ligand | BDBM50590039 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2194698 (CHEMBL5107058) |
|---|
| Ki | 31±n/a nM |
|---|
| Citation |  White, AM; DellsÚn, A; Larsson, N; Kaas, Q; Jansen, F; Plowright, AT; Knerr, L; Durek, T; Craik, DJ Late-Stage Functionalization with Cysteine Staples Generates Potent and Selective Melanocortin Receptor-1 Agonists. J Med Chem65:12956-12969 (2022) [PubMed] Article White, AM; DellsÚn, A; Larsson, N; Kaas, Q; Jansen, F; Plowright, AT; Knerr, L; Durek, T; Craik, DJ Late-Stage Functionalization with Cysteine Staples Generates Potent and Selective Melanocortin Receptor-1 Agonists. J Med Chem65:12956-12969 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Melanocortin receptor 4 |
|---|
| Name: | Melanocortin receptor 4 |
|---|
| Synonyms: | MC4-R | MC4R | MC4R_HUMAN | Melanocortin MC4 | Melanocortin receptor 4 (MC-4) | Melanocortin receptor 4 (MC4-R) | Melanocortin receptor 4 (MC4R) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36949.50 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P32245 |
|---|
| Residue: | 332 |
|---|
| Sequence: | MVNSTHRGMHTSLHLWNRSSYRLHSNASESLGKGYSDGGCYEQLFVSPEVFVTLGVISLL
ENILVIVAIAKNKNLHSPMYFFICSLAVADMLVSVSNGSETIVITLLNSTDTDAQSFTVN
IDNVIDSVICSSLLASICSLLSIAVDRYFTIFYALQYHNIMTVKRVGIIISCIWAACTVS
GILFIIYSDSSAVIICLITMFFTMLALMASLYVHMFLMARLHIKRIAVLPGTGAIRQGAN
MKGAITLTILIGVFVVCWAPFFLHLIFYISCPQNPYCVCFMSHFNLYLILIMCNSIIDPL
IYALRSQELRKTFKEIICCYPLGGLCDLSSRY
|
|
|
|---|
| BDBM50590039 |
|---|
| n/a |
|---|
| Name | BDBM50590039 |
|---|
| Synonyms: | CHEMBL5185775 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C107H150N38O22S2 |
|---|
| Mol. Mass. | 2384.707 |
|---|
| SMILES | [H][C@]12CSC\C(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)=N\OCC(=O)NCCOCCOCCOCCN=[N+]=[N-] |r| |
|---|
| Structure | 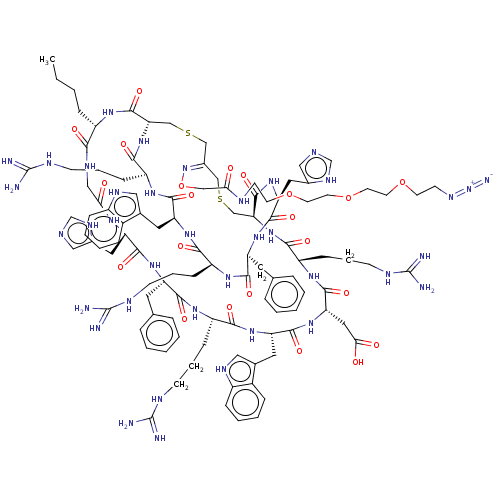
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 White, AM; DellsÚn, A; Larsson, N; Kaas, Q; Jansen, F; Plowright, AT; Knerr, L; Durek, T; Craik, DJ Late-Stage Functionalization with Cysteine Staples Generates Potent and Selective Melanocortin Receptor-1 Agonists. J Med Chem65:12956-12969 (2022) [PubMed] Article
White, AM; DellsÚn, A; Larsson, N; Kaas, Q; Jansen, F; Plowright, AT; Knerr, L; Durek, T; Craik, DJ Late-Stage Functionalization with Cysteine Staples Generates Potent and Selective Melanocortin Receptor-1 Agonists. J Med Chem65:12956-12969 (2022) [PubMed] Article