 Found 898 hits with Last Name = 'durek' and Initial = 't'
Found 898 hits with Last Name = 'durek' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590042
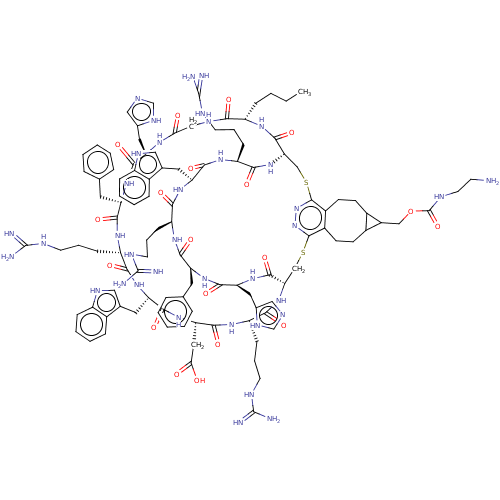
(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.00549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518241
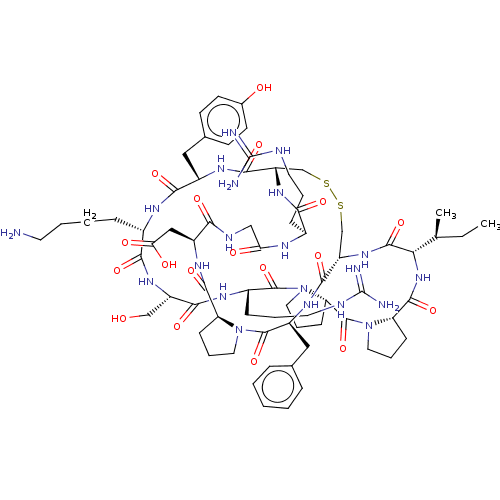
(CHEMBL4569923)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C72H107N21O18S2/c1-3-39(2)57-67(108)89-51-38-113-112-37-50(88-59(100)43(17-9-27-78-71(74)75)81-55(96)35-80-58(99)47(34-56(97)98)85-65(106)52-19-11-29-91(52)69(110)48(86-64(51)105)33-40-14-5-4-6-15-40)63(104)84-46(32-41-22-24-42(95)25-23-41)61(102)82-44(16-7-8-26-73)60(101)87-49(36-94)62(103)83-45(18-10-28-79-72(76)77)68(109)93-31-13-21-54(93)70(111)92-30-12-20-53(92)66(107)90-57/h4-6,14-15,22-25,39,43-54,57,94-95H,3,7-13,16-21,26-38,73H2,1-2H3,(H,80,99)(H,81,96)(H,82,102)(H,83,103)(H,84,104)(H,85,106)(H,86,105)(H,87,101)(H,88,100)(H,89,108)(H,90,107)(H,97,98)(H4,74,75,78)(H4,76,77,79)/t39-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518240

(CHEMBL4439523)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C72H107N19O18S2/c1-3-40(2)58-68(106)87-52-39-111-110-38-51(86-60(98)44(19-11-29-77-72(75)76)79-56(94)36-78-59(97)48(35-57(95)96)83-66(104)53-20-12-30-89(53)70(108)49(84-65(52)103)34-41-15-5-4-6-16-41)64(102)82-47(33-42-23-25-43(93)26-24-42)62(100)80-45(17-7-9-27-73)61(99)85-50(37-92)63(101)81-46(18-8-10-28-74)69(107)91-32-14-22-55(91)71(109)90-31-13-21-54(90)67(105)88-58/h4-6,15-16,23-26,40,44-55,58,92-93H,3,7-14,17-22,27-39,73-74H2,1-2H3,(H,78,97)(H,79,94)(H,80,100)(H,81,101)(H,82,102)(H,83,104)(H,84,103)(H,85,99)(H,86,98)(H,87,106)(H,88,105)(H,95,96)(H4,75,76,77)/t40-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590040
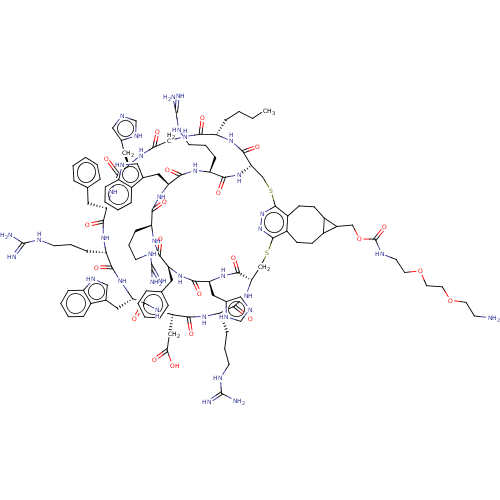
(CHEMBL5185945)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581303
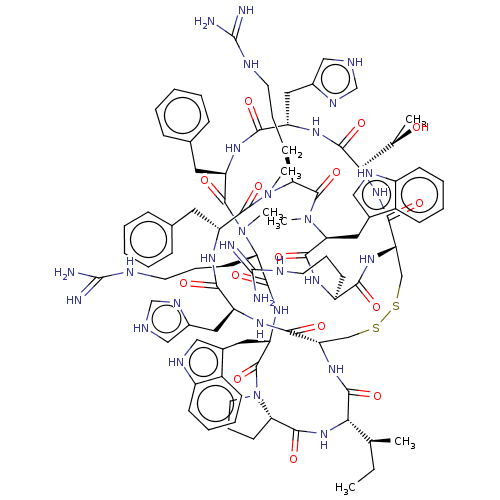
(CHEMBL5087859)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084
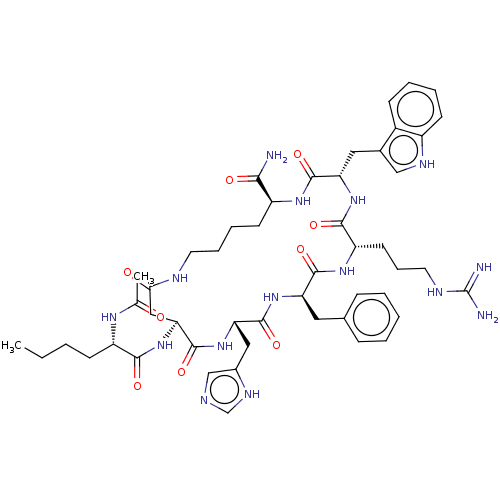
(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518249

(CHEMBL4588827)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)CC |r| Show InChI InChI=1S/C72H106N18O18S2/c1-5-39(3)57-68(105)85-51-38-110-109-37-50(84-60(97)44(19-12-28-76-72(74)75)78-55(93)35-77-59(96)47(34-56(94)95)81-66(103)52-20-13-29-88(52)69(106)48(82-65(51)102)33-41-16-8-7-9-17-41)64(101)80-46(32-42-23-25-43(92)26-24-42)62(99)79-45(18-10-11-27-73)61(98)83-49(36-91)63(100)87-58(40(4)6-2)71(108)90-31-15-22-54(90)70(107)89-30-14-21-53(89)67(104)86-57/h7-9,16-17,23-26,39-40,44-54,57-58,91-92H,5-6,10-15,18-22,27-38,73H2,1-4H3,(H,77,96)(H,78,93)(H,79,99)(H,80,101)(H,81,103)(H,82,102)(H,83,98)(H,84,97)(H,85,105)(H,86,104)(H,87,100)(H,94,95)(H4,74,75,76)/t39-,40-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581298
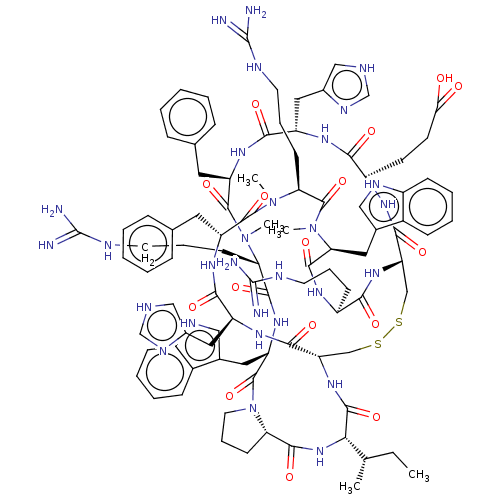
(CHEMBL5094168)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518247
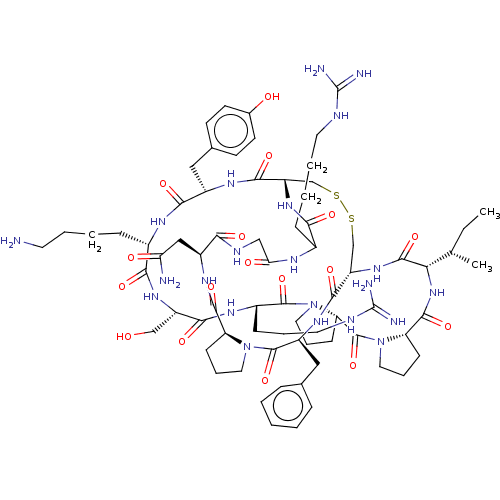
(CHEMBL4454304)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NC(CCCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C73H110N22O17S2/c1-3-40(2)58-68(109)91-52-39-114-113-38-51(90-60(101)44(18-8-10-28-80-72(76)77)83-57(99)36-82-59(100)48(35-56(75)98)87-66(107)53-20-12-30-93(53)70(111)49(88-65(52)106)34-41-15-5-4-6-16-41)64(105)86-47(33-42-23-25-43(97)26-24-42)62(103)84-45(17-7-9-27-74)61(102)89-50(37-96)63(104)85-46(19-11-29-81-73(78)79)69(110)95-32-14-22-55(95)71(112)94-31-13-21-54(94)67(108)92-58/h4-6,15-16,23-26,40,44-55,58,96-97H,3,7-14,17-22,27-39,74H2,1-2H3,(H2,75,98)(H,82,100)(H,83,99)(H,84,103)(H,85,104)(H,86,105)(H,87,107)(H,88,106)(H,89,102)(H,90,101)(H,91,109)(H,92,108)(H4,76,77,80)(H4,78,79,81)/t40-,44?,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590031
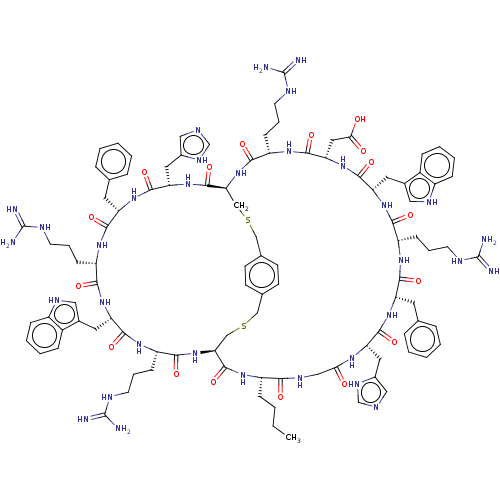
(CHEMBL5203986)Show SMILES [H][C@]12CSCc3ccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)cc3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590039
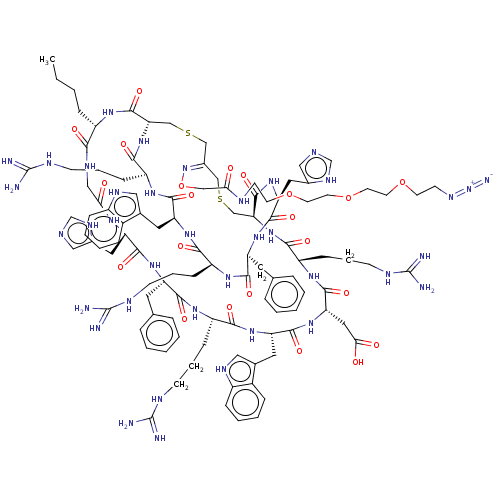
(CHEMBL5185775)Show SMILES [H][C@]12CSC\C(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)=N\OCC(=O)NCCOCCOCCOCCN=[N+]=[N-] |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590030
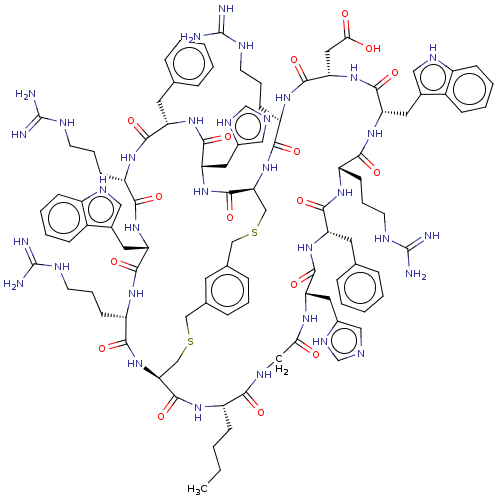
(CHEMBL5175487)Show SMILES [H][C@]12CSCc3cccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590047
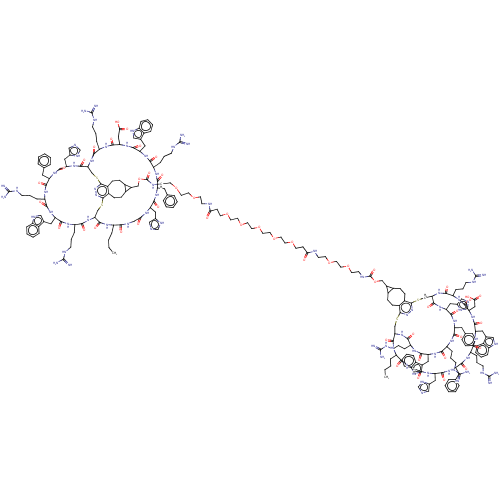
(CHEMBL5176443)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCNC(=O)CCOCCOCCOCCOCCOCCC(=O)NCCOCCOCCNC(=O)OCC4C5CCc6c(CCC45)c4SC[C@]5([H])NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc7c[nH]c8ccccc78)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc7ccccc7)NC(=O)[C@H](Cc7cnc[nH]7)NC(=O)CNC(=O)[C@H](CCCC)NC(=O)[C@]([H])(CSc6nn4)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4c[nH]c6ccccc46)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC5=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518253
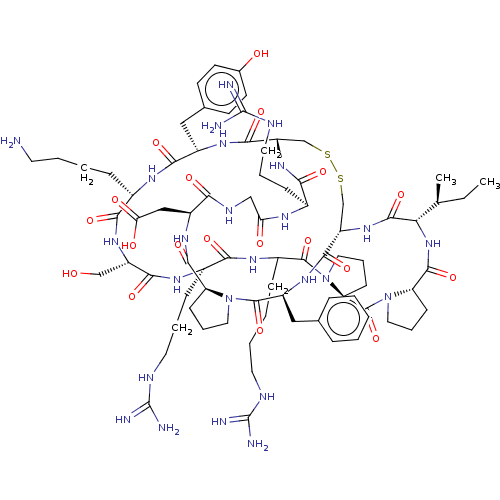
(CHEMBL4579797)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)C(CCCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C79H121N25O19S2/c1-3-43(2)62-73(120)100-56-42-125-124-41-55(99-64(111)47(20-11-31-88-78(83)84)91-60(107)39-90-63(110)52(38-61(108)109)96-71(118)57-22-13-33-102(57)75(122)53(97-70(56)117)37-44-16-5-4-6-17-44)69(116)95-51(36-45-25-27-46(106)28-26-45)67(114)92-48(18-7-9-29-80)66(113)98-54(40-105)68(115)93-49(21-12-32-89-79(85)86)65(112)94-50(19-8-10-30-87-77(81)82)74(121)104-35-15-24-59(104)76(123)103-34-14-23-58(103)72(119)101-62/h4-6,16-17,25-28,43,47-59,62,105-106H,3,7-15,18-24,29-42,80H2,1-2H3,(H,90,110)(H,91,107)(H,92,114)(H,93,115)(H,94,112)(H,95,116)(H,96,118)(H,97,117)(H,98,113)(H,99,111)(H,100,120)(H,101,119)(H,108,109)(H4,81,82,87)(H4,83,84,88)(H4,85,86,89)/t43-,47-,48-,49-,50?,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518252

(CHEMBL4592533)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C72H107N19O17S2/c1-3-41(2)58-68(105)87-52-40-110-109-39-51(86-60(97)44(24-14-30-77-72(75)76)79-56(93)37-78-59(96)48(36-57(94)95)83-66(103)53-25-15-31-89(53)70(107)49(84-65(52)102)35-43-20-8-5-9-21-43)64(101)82-47(34-42-18-6-4-7-19-42)62(99)80-45(22-10-12-28-73)61(98)85-50(38-92)63(100)81-46(23-11-13-29-74)69(106)91-33-17-27-55(91)71(108)90-32-16-26-54(90)67(104)88-58/h4-9,18-21,41,44-55,58,92H,3,10-17,22-40,73-74H2,1-2H3,(H,78,96)(H,79,93)(H,80,99)(H,81,100)(H,82,101)(H,83,103)(H,84,102)(H,85,98)(H,86,97)(H,87,105)(H,88,104)(H,94,95)(H4,75,76,77)/t41-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590042

(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590041
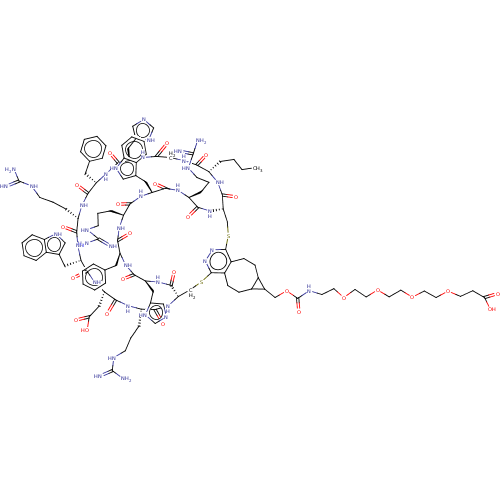
(CHEMBL5207936)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCOCCOCCC(O)=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518243
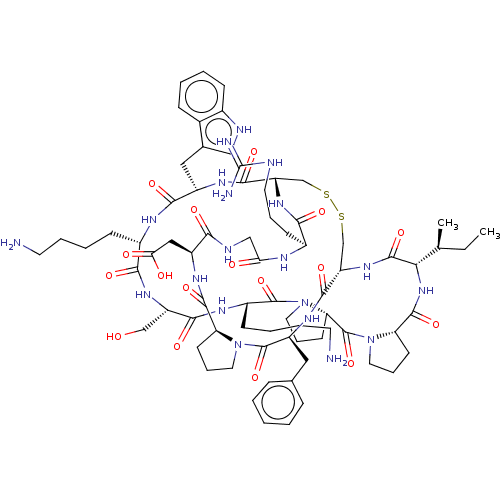
(CHEMBL4435567)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C74H108N20O17S2/c1-3-41(2)60-70(108)90-54-40-113-112-39-53(89-62(100)46(23-13-29-79-74(77)78)82-58(96)37-81-61(99)50(35-59(97)98)86-68(106)55-24-14-30-92(55)72(110)51(87-67(54)105)33-42-17-5-4-6-18-42)66(104)85-49(34-43-36-80-45-20-8-7-19-44(43)45)64(102)83-47(21-9-11-27-75)63(101)88-52(38-95)65(103)84-48(22-10-12-28-76)71(109)94-32-16-26-57(94)73(111)93-31-15-25-56(93)69(107)91-60/h4-8,17-20,36,41,46-57,60,80,95H,3,9-16,21-35,37-40,75-76H2,1-2H3,(H,81,99)(H,82,96)(H,83,102)(H,84,103)(H,85,104)(H,86,106)(H,87,105)(H,88,101)(H,89,100)(H,90,108)(H,91,107)(H,97,98)(H4,77,78,79)/t41-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,60-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518255
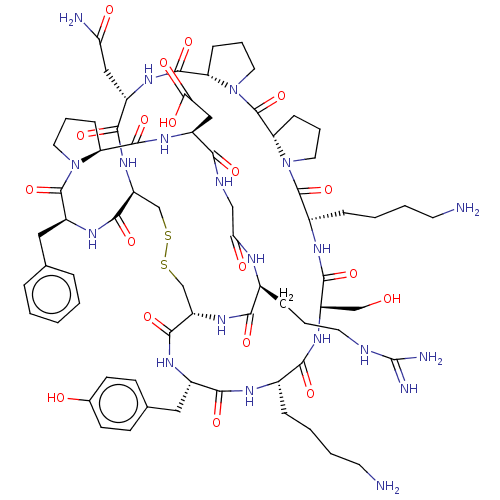
(CHEMBL4443353)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C70H102N20O19S2/c71-24-6-4-14-42-59(99)85-48(35-91)62(102)80-43(15-5-7-25-72)67(107)90-29-11-19-53(90)69(109)89-28-10-18-52(89)66(106)82-45(32-54(73)93)61(101)87-50-37-111-110-36-49(63(103)81-44(60(100)79-42)30-39-20-22-40(92)23-21-39)86-58(98)41(16-8-26-76-70(74)75)78-55(94)34-77-57(97)46(33-56(95)96)83-65(105)51-17-9-27-88(51)68(108)47(84-64(50)104)31-38-12-2-1-3-13-38/h1-3,12-13,20-23,41-53,91-92H,4-11,14-19,24-37,71-72H2,(H2,73,93)(H,77,97)(H,78,94)(H,79,100)(H,80,102)(H,81,103)(H,82,106)(H,83,105)(H,84,104)(H,85,99)(H,86,98)(H,87,101)(H,95,96)(H4,74,75,76)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590046
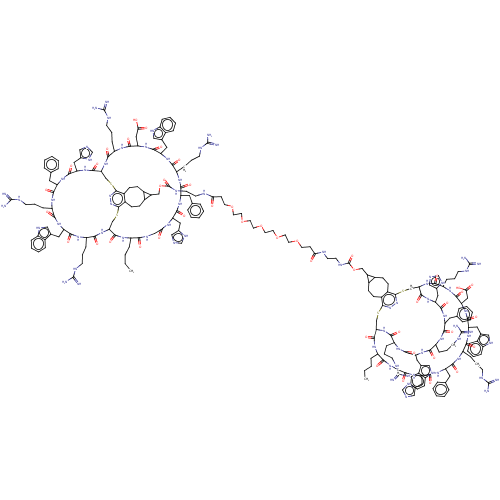
(CHEMBL5181812)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCNC(=O)CCOCCOCCOCCOCCOCCC(=O)NCCNC(=O)OCC4C5CCc6c(CCC45)c4SC[C@]5([H])NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc7c[nH]c8ccccc78)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc7ccccc7)NC(=O)[C@H](Cc7cnc[nH]7)NC(=O)CNC(=O)[C@H](CCCC)NC(=O)[C@]([H])(CSc6nn4)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4c[nH]c6ccccc46)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC5=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581296
(CHEMBL5092761)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590037
(CHEMBL5191309)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)nn3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581313
(CHEMBL5087839)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc3ccccc3c2)C(=O)N[C@@H](C)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590032
(CHEMBL5178164)Show SMILES [H][C@]12CSc3c(F)c(F)c(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c(F)c3F |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581297
(CHEMBL5091236)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50590042

(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581311
(CHEMBL5083551)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518239

(CHEMBL4453437)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)NC(CCCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C74H111N21O18S2/c1-3-41(2)59-69(110)91-53-40-115-114-39-52(90-61(102)45(18-8-10-30-80-73(76)77)83-57(98)37-82-60(101)47(27-28-58(99)100)85-67(108)54-20-12-32-93(54)71(112)50(88-66(53)107)36-42-15-5-4-6-16-42)65(106)87-49(35-43-23-25-44(97)26-24-43)63(104)84-46(17-7-9-29-75)62(103)89-51(38-96)64(105)86-48(19-11-31-81-74(78)79)70(111)95-34-14-22-56(95)72(113)94-33-13-21-55(94)68(109)92-59/h4-6,15-16,23-26,41,45-56,59,96-97H,3,7-14,17-22,27-40,75H2,1-2H3,(H,82,101)(H,83,98)(H,84,104)(H,85,108)(H,86,105)(H,87,106)(H,88,107)(H,89,103)(H,90,102)(H,91,110)(H,92,109)(H,99,100)(H4,76,77,80)(H4,78,79,81)/t41-,45?,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,59-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581306

(CHEMBL5077095)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518245
(CHEMBL4471930)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C72H108N22O17S2/c1-3-39(2)57-67(108)90-51-38-113-112-37-50(89-59(100)43(17-9-27-79-71(75)76)82-56(98)35-81-58(99)47(34-55(74)97)86-65(106)52-19-11-29-92(52)69(110)48(87-64(51)105)33-40-14-5-4-6-15-40)63(104)85-46(32-41-22-24-42(96)25-23-41)61(102)83-44(16-7-8-26-73)60(101)88-49(36-95)62(103)84-45(18-10-28-80-72(77)78)68(109)94-31-13-21-54(94)70(111)93-30-12-20-53(93)66(107)91-57/h4-6,14-15,22-25,39,43-54,57,95-96H,3,7-13,16-21,26-38,73H2,1-2H3,(H2,74,97)(H,81,99)(H,82,98)(H,83,102)(H,84,103)(H,85,104)(H,86,106)(H,87,105)(H,88,101)(H,89,100)(H,90,108)(H,91,107)(H4,75,76,79)(H4,77,78,80)/t39-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC4R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581299
(CHEMBL5075506)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590033
(CHEMBL5170533)Show SMILES [H][C@]12CSCCCSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581314

(CHEMBL5090946)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](C)C(=O)N2)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581310
(CHEMBL5077144)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581302
(CHEMBL5075712)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590029
(CHEMBL5192329)Show SMILES [H][C@]12CSCc3ccccc3CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590038
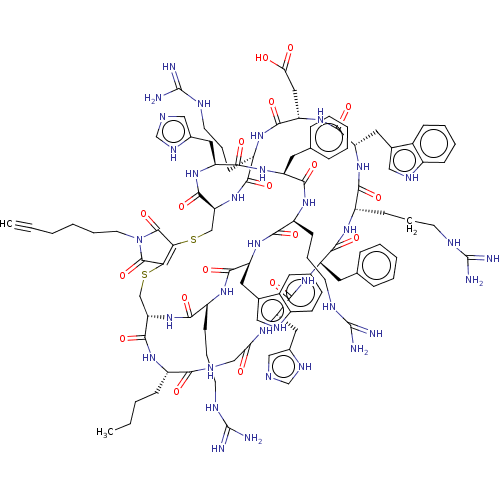
(CHEMBL5172738)Show SMILES [H][C@]12CSC3=C(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)C(=O)N(CCCCC#C)C3=O |r,c:4| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518251

(CHEMBL4515559)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C71H104N20O19S2/c72-26-6-4-14-43-60(100)86-49(36-92)63(103)82-45(15-5-7-27-73)68(108)91-31-11-19-54(91)70(110)90-30-10-18-53(90)66(106)81-44(24-25-55(74)94)61(101)88-51-38-112-111-37-50(64(104)83-46(62(102)80-43)32-40-20-22-41(93)23-21-40)87-59(99)42(16-8-28-77-71(75)76)79-56(95)35-78-58(98)47(34-57(96)97)84-67(107)52-17-9-29-89(52)69(109)48(85-65(51)105)33-39-12-2-1-3-13-39/h1-3,12-13,20-23,42-54,92-93H,4-11,14-19,24-38,72-73H2,(H2,74,94)(H,78,98)(H,79,95)(H,80,102)(H,81,106)(H,82,103)(H,83,104)(H,84,107)(H,85,105)(H,86,100)(H,87,99)(H,88,101)(H,96,97)(H4,75,76,77)/t42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50389769
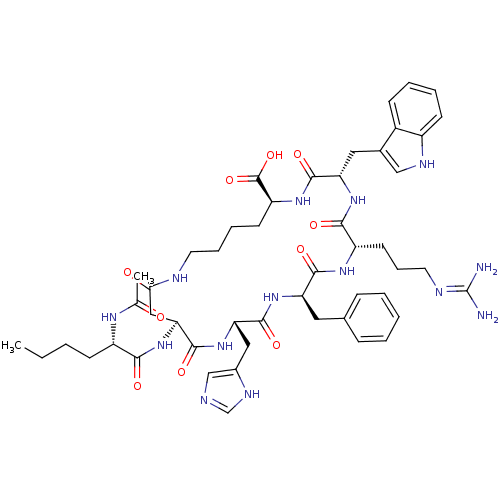
(BREMELANOTIDE)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(O)=O |r,wU:61.64,50.52,39.41,25.25,4.4,wD:12.11,21.75,(-11.27,-.98,;-11.98,-2.34,;-11.15,-3.64,;-9.61,-3.57,;-8.78,-4.87,;-9.49,-6.24,;-11.03,-6.31,;-11.73,-7.67,;-11.86,-5.01,;-7.24,-4.8,;-6.41,-6.1,;-6.53,-3.43,;-4.99,-3.36,;-4.15,-4.65,;-4.16,-6.3,;-5.38,-7.46,;9.13,-7.95,;9.83,-6.57,;8.99,-5.29,;9.68,-3.91,;8.85,-2.63,;9.54,-1.25,;8.7,.04,;7.17,-.04,;6.47,-1.42,;6.33,1.25,;7.03,2.62,;8.56,2.7,;9.53,1.55,;10.96,2.1,;10.89,3.65,;11.98,4.73,;11.57,6.22,;10.08,6.61,;9,5.52,;9.4,4.04,;4.79,1.16,;3.95,2.46,;4.65,3.83,;2.41,2.38,;1.57,3.67,;2.27,5.04,;3.81,5.12,;4.51,6.49,;6.04,6.57,;6.88,5.28,;6.74,7.95,;1.71,1,;.18,.92,;-.66,2.21,;-.52,-.45,;.32,-1.74,;1.86,-1.66,;2.69,-2.95,;4.24,-2.86,;4.93,-1.49,;4.09,-.2,;2.55,-.28,;-2.06,-.53,;-2.9,.76,;-2.2,2.13,;-4.44,.67,;-5.28,1.97,;-4.58,3.34,;-5.29,4.71,;-4.2,5.81,;-2.82,5.1,;-3.06,3.57,;-5.14,-.69,;-4.3,-1.99,;-2.76,-1.9,;11.08,-1.17,;11.92,-2.46,;11.78,.2,)| Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-35(58-29(2)65)43(67)64-41-25-42(66)54-20-11-10-18-37(49(73)74)60-46(70)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(68)36(19-12-21-55-50(51)52)59-45(69)38(22-30-13-6-5-7-14-30)61-47(71)40(63-48(41)72)24-32-27-53-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25H2,1-2H3,(H,53,57)(H,54,66)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,64,67)(H,73,74)(H4,51,52,55)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50389769

(BREMELANOTIDE)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(O)=O |r,wU:61.64,50.52,39.41,25.25,4.4,wD:12.11,21.75,(-11.27,-.98,;-11.98,-2.34,;-11.15,-3.64,;-9.61,-3.57,;-8.78,-4.87,;-9.49,-6.24,;-11.03,-6.31,;-11.73,-7.67,;-11.86,-5.01,;-7.24,-4.8,;-6.41,-6.1,;-6.53,-3.43,;-4.99,-3.36,;-4.15,-4.65,;-4.16,-6.3,;-5.38,-7.46,;9.13,-7.95,;9.83,-6.57,;8.99,-5.29,;9.68,-3.91,;8.85,-2.63,;9.54,-1.25,;8.7,.04,;7.17,-.04,;6.47,-1.42,;6.33,1.25,;7.03,2.62,;8.56,2.7,;9.53,1.55,;10.96,2.1,;10.89,3.65,;11.98,4.73,;11.57,6.22,;10.08,6.61,;9,5.52,;9.4,4.04,;4.79,1.16,;3.95,2.46,;4.65,3.83,;2.41,2.38,;1.57,3.67,;2.27,5.04,;3.81,5.12,;4.51,6.49,;6.04,6.57,;6.88,5.28,;6.74,7.95,;1.71,1,;.18,.92,;-.66,2.21,;-.52,-.45,;.32,-1.74,;1.86,-1.66,;2.69,-2.95,;4.24,-2.86,;4.93,-1.49,;4.09,-.2,;2.55,-.28,;-2.06,-.53,;-2.9,.76,;-2.2,2.13,;-4.44,.67,;-5.28,1.97,;-4.58,3.34,;-5.29,4.71,;-4.2,5.81,;-2.82,5.1,;-3.06,3.57,;-5.14,-.69,;-4.3,-1.99,;-2.76,-1.9,;11.08,-1.17,;11.92,-2.46,;11.78,.2,)| Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-35(58-29(2)65)43(67)64-41-25-42(66)54-20-11-10-18-37(49(73)74)60-46(70)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(68)36(19-12-21-55-50(51)52)59-45(69)38(22-30-13-6-5-7-14-30)61-47(71)40(63-48(41)72)24-32-27-53-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25H2,1-2H3,(H,53,57)(H,54,66)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,64,67)(H,73,74)(H4,51,52,55)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50389769

(BREMELANOTIDE)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(O)=O |r,wU:61.64,50.52,39.41,25.25,4.4,wD:12.11,21.75,(-11.27,-.98,;-11.98,-2.34,;-11.15,-3.64,;-9.61,-3.57,;-8.78,-4.87,;-9.49,-6.24,;-11.03,-6.31,;-11.73,-7.67,;-11.86,-5.01,;-7.24,-4.8,;-6.41,-6.1,;-6.53,-3.43,;-4.99,-3.36,;-4.15,-4.65,;-4.16,-6.3,;-5.38,-7.46,;9.13,-7.95,;9.83,-6.57,;8.99,-5.29,;9.68,-3.91,;8.85,-2.63,;9.54,-1.25,;8.7,.04,;7.17,-.04,;6.47,-1.42,;6.33,1.25,;7.03,2.62,;8.56,2.7,;9.53,1.55,;10.96,2.1,;10.89,3.65,;11.98,4.73,;11.57,6.22,;10.08,6.61,;9,5.52,;9.4,4.04,;4.79,1.16,;3.95,2.46,;4.65,3.83,;2.41,2.38,;1.57,3.67,;2.27,5.04,;3.81,5.12,;4.51,6.49,;6.04,6.57,;6.88,5.28,;6.74,7.95,;1.71,1,;.18,.92,;-.66,2.21,;-.52,-.45,;.32,-1.74,;1.86,-1.66,;2.69,-2.95,;4.24,-2.86,;4.93,-1.49,;4.09,-.2,;2.55,-.28,;-2.06,-.53,;-2.9,.76,;-2.2,2.13,;-4.44,.67,;-5.28,1.97,;-4.58,3.34,;-5.29,4.71,;-4.2,5.81,;-2.82,5.1,;-3.06,3.57,;-5.14,-.69,;-4.3,-1.99,;-2.76,-1.9,;11.08,-1.17,;11.92,-2.46,;11.78,.2,)| Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-35(58-29(2)65)43(67)64-41-25-42(66)54-20-11-10-18-37(49(73)74)60-46(70)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(68)36(19-12-21-55-50(51)52)59-45(69)38(22-30-13-6-5-7-14-30)61-47(71)40(63-48(41)72)24-32-27-53-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25H2,1-2H3,(H,53,57)(H,54,66)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,64,67)(H,73,74)(H4,51,52,55)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50263818
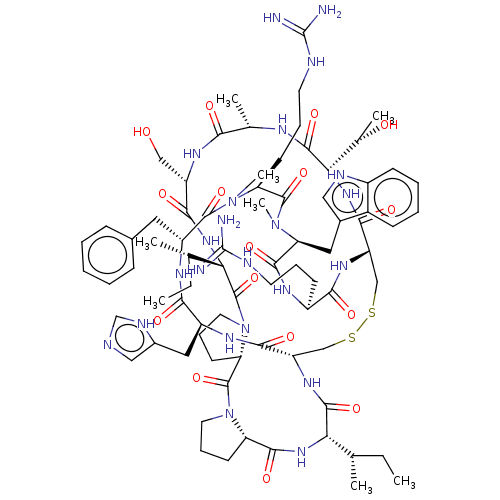
(CHEMBL4089162)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C78H115N23O16S2/c1-9-41(3)60-71(112)94-54-38-118-119-39-55(68(109)97-62(44(6)103)72(113)88-43(5)63(104)92-53(37-102)66(107)96-61(42(4)10-2)76(117)101-31-19-27-58(101)75(116)100-30-18-26-56(100)69(110)95-60)93-64(105)50(24-16-28-84-77(79)80)89-70(111)59(33-46-35-86-49-23-15-14-22-48(46)49)99(8)74(115)57(25-17-29-85-78(81)82)98(7)73(114)52(32-45-20-12-11-13-21-45)91-65(106)51(90-67(54)108)34-47-36-83-40-87-47/h11-15,20-23,35-36,40-44,50-62,86,102-103H,9-10,16-19,24-34,37-39H2,1-8H3,(H,83,87)(H,88,113)(H,89,111)(H,90,108)(H,91,106)(H,92,104)(H,93,105)(H,94,112)(H,95,110)(H,96,107)(H,97,109)(H4,79,80,84)(H4,81,82,85)/t41-,42-,43-,44-,50-,51-,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
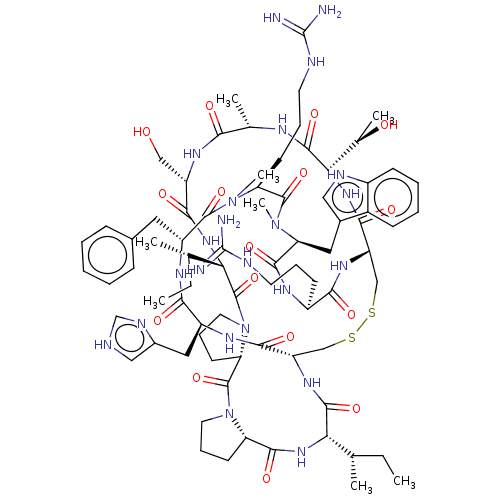
(Homo sapiens (Human)) | BDBM50581301
(CHEMBL5093939)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O)[C@@H](C)CC |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50518244
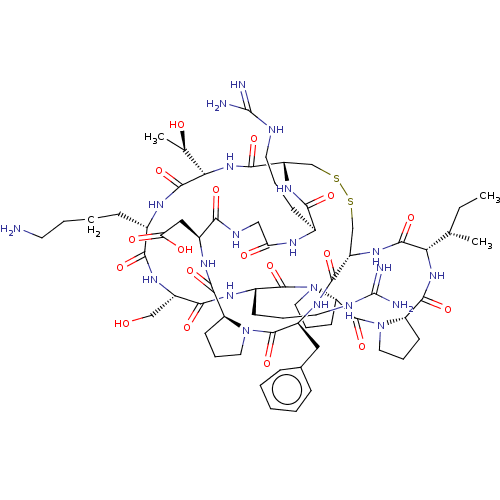
(CHEMBL4550948)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O |r| Show InChI InChI=1S/C67H105N21O18S2/c1-4-35(2)51-61(102)83-44-33-107-108-34-45(82-54(95)38(18-10-24-73-66(69)70)76-49(91)31-75-53(94)41(30-50(92)93)79-59(100)46-20-12-26-86(46)64(105)42(80-57(44)98)29-37-15-6-5-7-16-37)58(99)85-52(36(3)90)62(103)77-39(17-8-9-23-68)55(96)81-43(32-89)56(97)78-40(19-11-25-74-67(71)72)63(104)88-28-14-22-48(88)65(106)87-27-13-21-47(87)60(101)84-51/h5-7,15-16,35-36,38-48,51-52,89-90H,4,8-14,17-34,68H2,1-3H3,(H,75,94)(H,76,91)(H,77,103)(H,78,97)(H,79,100)(H,80,98)(H,81,96)(H,82,95)(H,83,102)(H,84,101)(H,85,99)(H,92,93)(H4,69,70,73)(H4,71,72,74)/t35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins |
J Med Chem 62: 552-560 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01139
BindingDB Entry DOI: 10.7270/Q2MS3X3S |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590034
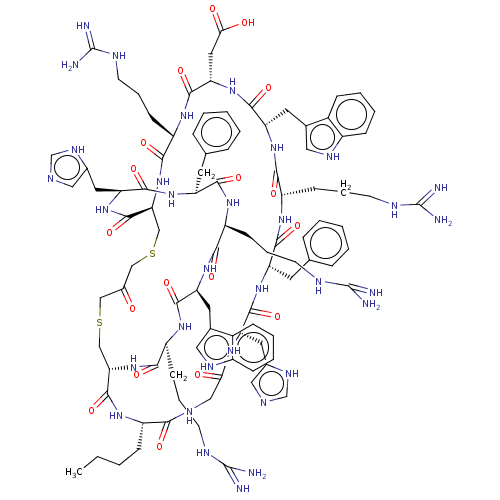
(CHEMBL5190042)Show SMILES [H][C@]12CSCC(=O)CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data