| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Somatostatin receptor type 5 |
|---|
| Ligand | BDBM50592171 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2203288 (CHEMBL5115996) |
|---|
| EC50 | 19±n/a nM |
|---|
| Citation |  Zhao, J; Wang, S; Hee Kim, S; Han, S; Rico-Bautista, E; Sturchler, E; Nguyen, J; Tan, H; Staley, C; Karin Kusnetzow, A; Betz, SF; Johns, M; Goulet, L; Luo, R; Fowler, M; Athanacio, J; Markison, S; Scott Struthers, R; Zhu, Y Discovery of 4-(3-aminopyrrolidinyl)-3-aryl-5-(benzimidazol-2-yl)-pyridines as potent and selective SST5 agonists for the treatment of congenital hyperinsulinism. Bioorg Med Chem Lett71:0 (2022) [PubMed] Article Zhao, J; Wang, S; Hee Kim, S; Han, S; Rico-Bautista, E; Sturchler, E; Nguyen, J; Tan, H; Staley, C; Karin Kusnetzow, A; Betz, SF; Johns, M; Goulet, L; Luo, R; Fowler, M; Athanacio, J; Markison, S; Scott Struthers, R; Zhu, Y Discovery of 4-(3-aminopyrrolidinyl)-3-aryl-5-(benzimidazol-2-yl)-pyridines as potent and selective SST5 agonists for the treatment of congenital hyperinsulinism. Bioorg Med Chem Lett71:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Somatostatin receptor type 5 |
|---|
| Name: | Somatostatin receptor type 5 |
|---|
| Synonyms: | SOMATOSTATIN SST5 | SS-5-R | SS5-R | SS5R | SSR5_HUMAN | SSTR5 | Somatostatin receptor type 5 (SSTR5) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 39218.02 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35346 |
|---|
| Residue: | 364 |
|---|
| Sequence: | MEPLFPASTPSWNASSPGAASGGGDNRTLVGPAPSAGARAVLVPVLYLLVCAAGLGGNTL
VIYVVLRFAKMKTVTNIYILNLAVADVLYMLGLPFLATQNAASFWPFGPVLCRLVMTLDG
VNQFTSVFCLTVMSVDRYLAVVHPLSSARWRRPRVAKLASAAAWVLSLCMSLPLLVFADV
QEGGTCNASWPEPVGLWGAVFIIYTAVLGFFAPLLVICLCYLLIVVKVRAAGVRVGCVRR
RSERKVTRMVLVVVLVFAGCWLPFFTVNIVNLAVALPQEPASAGLYFFVVILSYANSCAN
PVLYGFLSDNFRQSFQKVLCLRKGSGAKDADATEPRPDRIRQQQEATPPAHRAAANGLMQ
TSKL
|
|
|
|---|
| BDBM50592171 |
|---|
| n/a |
|---|
| Name | BDBM50592171 |
|---|
| Synonyms: | CHEMBL5190580 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H21FN6 |
|---|
| Mol. Mass. | 412.4621 |
|---|
| SMILES | Cc1cc(F)cc(c1)-c1cncc(-c2nc3c(cccc3[nH]2)C#N)c1N1CC(CN)C1 |
|---|
| Structure | 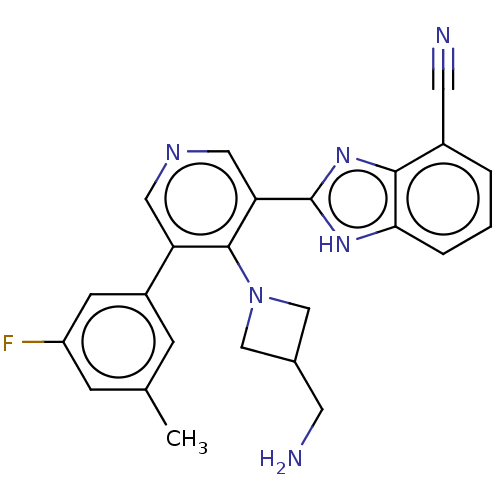
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhao, J; Wang, S; Hee Kim, S; Han, S; Rico-Bautista, E; Sturchler, E; Nguyen, J; Tan, H; Staley, C; Karin Kusnetzow, A; Betz, SF; Johns, M; Goulet, L; Luo, R; Fowler, M; Athanacio, J; Markison, S; Scott Struthers, R; Zhu, Y Discovery of 4-(3-aminopyrrolidinyl)-3-aryl-5-(benzimidazol-2-yl)-pyridines as potent and selective SST5 agonists for the treatment of congenital hyperinsulinism. Bioorg Med Chem Lett71:0 (2022) [PubMed] Article
Zhao, J; Wang, S; Hee Kim, S; Han, S; Rico-Bautista, E; Sturchler, E; Nguyen, J; Tan, H; Staley, C; Karin Kusnetzow, A; Betz, SF; Johns, M; Goulet, L; Luo, R; Fowler, M; Athanacio, J; Markison, S; Scott Struthers, R; Zhu, Y Discovery of 4-(3-aminopyrrolidinyl)-3-aryl-5-(benzimidazol-2-yl)-pyridines as potent and selective SST5 agonists for the treatment of congenital hyperinsulinism. Bioorg Med Chem Lett71:0 (2022) [PubMed] Article