| Reaction Details |
|---|
 | Report a problem with these data |
| Target | High affinity nerve growth factor receptor |
|---|
| Ligand | BDBM185674 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2220455 (CHEMBL5133789) |
|---|
| IC50 | 5.5±n/a nM |
|---|
| Citation |  Dokla, EME; Abdel-Aziz, AK; Milik, SN; McPhillie, MJ; Minucci, S; Abouzid, KAM Discovery of a benzimidazole-based dual FLT3/TrKA inhibitor targeting acute myeloid leukemia. Bioorg Med Chem56:0 (2022) [PubMed] Article Dokla, EME; Abdel-Aziz, AK; Milik, SN; McPhillie, MJ; Minucci, S; Abouzid, KAM Discovery of a benzimidazole-based dual FLT3/TrKA inhibitor targeting acute myeloid leukemia. Bioorg Med Chem56:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| High affinity nerve growth factor receptor |
|---|
| Name: | High affinity nerve growth factor receptor |
|---|
| Synonyms: | 2.7.10.1 | MTC | NTRK1 | NTRK1/NTRK2 | NTRK1_HUMAN | Nerve growth factor receptor Trk-A | Neurotrophic tyrosine kinase receptor type 1 | Neurotrophic tyrosine kinase receptor type 1 (TrkA) | Synonyms=MTC | TRK | TRK1-transforming tyrosine kinase protein | TRKA | TRKA GN | TRKA GN | Trk-A | Tropomyosin alpha-3 chain/High affinity nerve growth factor receptor | Tropomyosin-related kinase A | Tropomyosin-related kinase A (TrkA) | Tyrosine kinase receptor | Tyrosine kinase receptor (Trk) | Tyrosine kinase receptor A | Tyrosine kinase receptor A (Trk A) | Tyrosine kinase receptor A (Trk-A) | Tyrosine kinase receptor A (TrkA) | gp140trk | p140-TrkA |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 87498.18 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04629 |
|---|
| Residue: | 796 |
|---|
| Sequence: | MLRGGRRGQLGWHSWAAGPGSLLAWLILASAGAAPCPDACCPHGSSGLRCTRDGALDSLH
HLPGAENLTELYIENQQHLQHLELRDLRGLGELRNLTIVKSGLRFVAPDAFHFTPRLSRL
NLSFNALESLSWKTVQGLSLQELVLSGNPLHCSCALRWLQRWEEEGLGGVPEQKLQCHGQ
GPLAHMPNASCGVPTLKVQVPNASVDVGDDVLLRCQVEGRGLEQAGWILTELEQSATVMK
SGGLPSLGLTLANVTSDLNRKNVTCWAENDVGRAEVSVQVNVSFPASVQLHTAVEMHHWC
IPFSVDGQPAPSLRWLFNGSVLNETSFIFTEFLEPAANETVRHGCLRLNQPTHVNNGNYT
LLAANPFGQASASIMAAFMDNPFEFNPEDPIPVSFSPVDTNSTSGDPVEKKDETPFGVSV
AVGLAVFACLFLSTLLLVLNKCGRRNKFGINRPAVLAPEDGLAMSLHFMTLGGSSLSPTE
GKGSGLQGHIIENPQYFSDACVHHIKRRDIVLKWELGEGAFGKVFLAECHNLLPEQDKML
VAVKALKEASESARQDFQREAELLTMLQHQHIVRFFGVCTEGRPLLMVFEYMRHGDLNRF
LRSHGPDAKLLAGGEDVAPGPLGLGQLLAVASQVAAGMVYLAGLHFVHRDLATRNCLVGQ
GLVVKIGDFGMSRDIYSTDYYRVGGRTMLPIRWMPPESILYRKFTTESDVWSFGVVLWEI
FTYGKQPWYQLSNTEAIDCITQGRELERPRACPPEVYAIMRGCWQREPQQRHSIKDVHAR
LQALAQAPPVYLDVLG
|
|
|
|---|
| BDBM185674 |
|---|
| n/a |
|---|
| Name | BDBM185674 |
|---|
| Synonyms: | 4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide | Rebastinib |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H28FN7O3 |
|---|
| Mol. Mass. | 553.5868 |
|---|
| SMILES | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 |
|---|
| Structure | 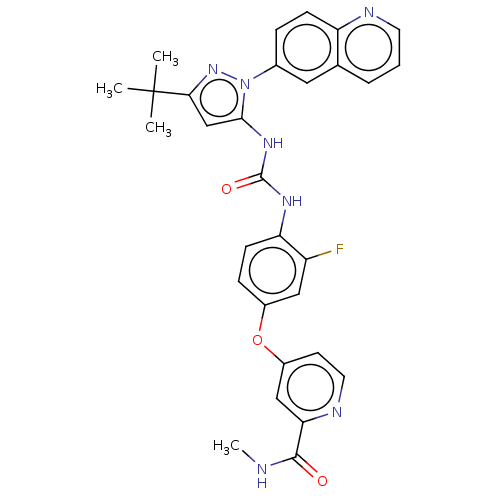
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dokla, EME; Abdel-Aziz, AK; Milik, SN; McPhillie, MJ; Minucci, S; Abouzid, KAM Discovery of a benzimidazole-based dual FLT3/TrKA inhibitor targeting acute myeloid leukemia. Bioorg Med Chem56:0 (2022) [PubMed] Article
Dokla, EME; Abdel-Aziz, AK; Milik, SN; McPhillie, MJ; Minucci, S; Abouzid, KAM Discovery of a benzimidazole-based dual FLT3/TrKA inhibitor targeting acute myeloid leukemia. Bioorg Med Chem56:0 (2022) [PubMed] Article